Methods and Compositions for the Diagnosis of Cancer Susceptibilities and Defective DNA Repair Mechanisms and Treatment Thereof
a technology of dna repair and cancer susceptibility, applied in the direction of immunoglobulins against animals/humans, peptide sources, instruments, etc., can solve the problems of complex diagnosis of fa, inability to differentiate fa carriers from the general population, and inability to detect fa carriers. to achieve the effect of reducing intracellular drug levels, reducing inward transport, and increasing drug inactivation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Experimental Protocols Used in Examples 2-8
[0240]Cell Lines and Culture Conditions. Epstein-Barr virus (EBV) transformed lymphoblasts were maintained in RPMI media supplemented with 15% heat-inactivated fetal calf serum (FCS) and grown in a humidified 5% CO2-containing atmosphere at 37° C. A control lymphoblast line (PD7) and FA lymphoblast lines (FA-A (HSC72), FA-C(PD-4), FA-D (PD-20), FA-F (EUFA121), and FA-G (EUFA316)) have been previously described (de Winter et al., Nat. Genet., (1998) Vol. 20, pp. 281-283) (Whitney et al., Nat. Genet., (1995) Vol. 11, pp. 341-343) (Yamashita et al., P.N.A.S., (1994) Vol. 91, pp. 6712-6716) (de Winter et al., Am. J. Hum. Genet., (2001), Vol. 57, pp. 1306-1308). PD81 is a lymphoblast cell line from an FA-A patient. The SV40-transformed FA fibroblasts, GM6914, PD426, FAG326SV and PD20F, as well as HeLa cells, were grown in DMEM supplemented with 15% FCS. FA cells (both lymphoblasts and fibroblasts) were functionally complemented with pMMP retrovi...
example 2
The FA Genes Interact in a Common Cellular Pathway
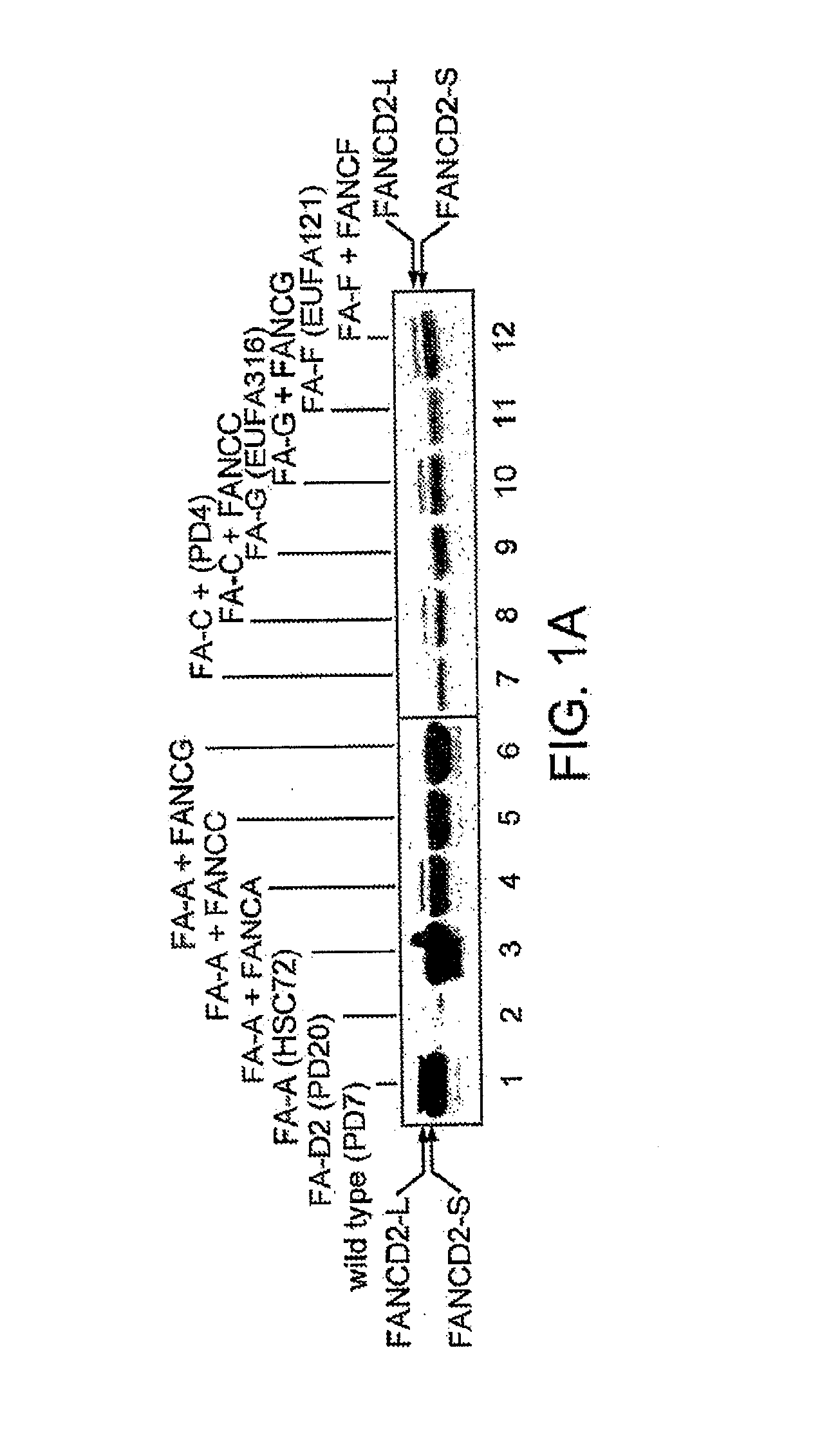
[0253]Normal lymphoblasts express two isoforms of the FANCD2 protein, a short form (FANCD2-S, 155 kD) and a long form (FANCD2-L, 162 kD). FIG. 1 shows what happened when whole cell extracts were prepared from a lymphoblast line and cellular proteins were immunoprecipitated with an anti-FANCD2 antiserum. Normal wild type cells expressed two isoforms of the FANCD2 protein—a low molecular weight isoform FANCD2-S (155 kD isoform) and a high molecular weight isoform (FANCD2-L) (162 kD isoform). FANCD2-S is the primary translation product of the cloned FANCD2 cDNA. We next evaluated a large series of FA lymphoblasts and fibroblasts for expression of the FANCD2 isoforms (Table 5). Correction of these FA cell lines with the corresponding FA cDNA resulted in functional complementation and restoration of the high molecular weight isoform, FANCD2-L.
[0254]As previously described, FA cells are sensitive to the DNA crosslinking agent, MMC, and in ...
example 3
The FA Protein Complex is Required for the Monoubiquitination of FANCD2
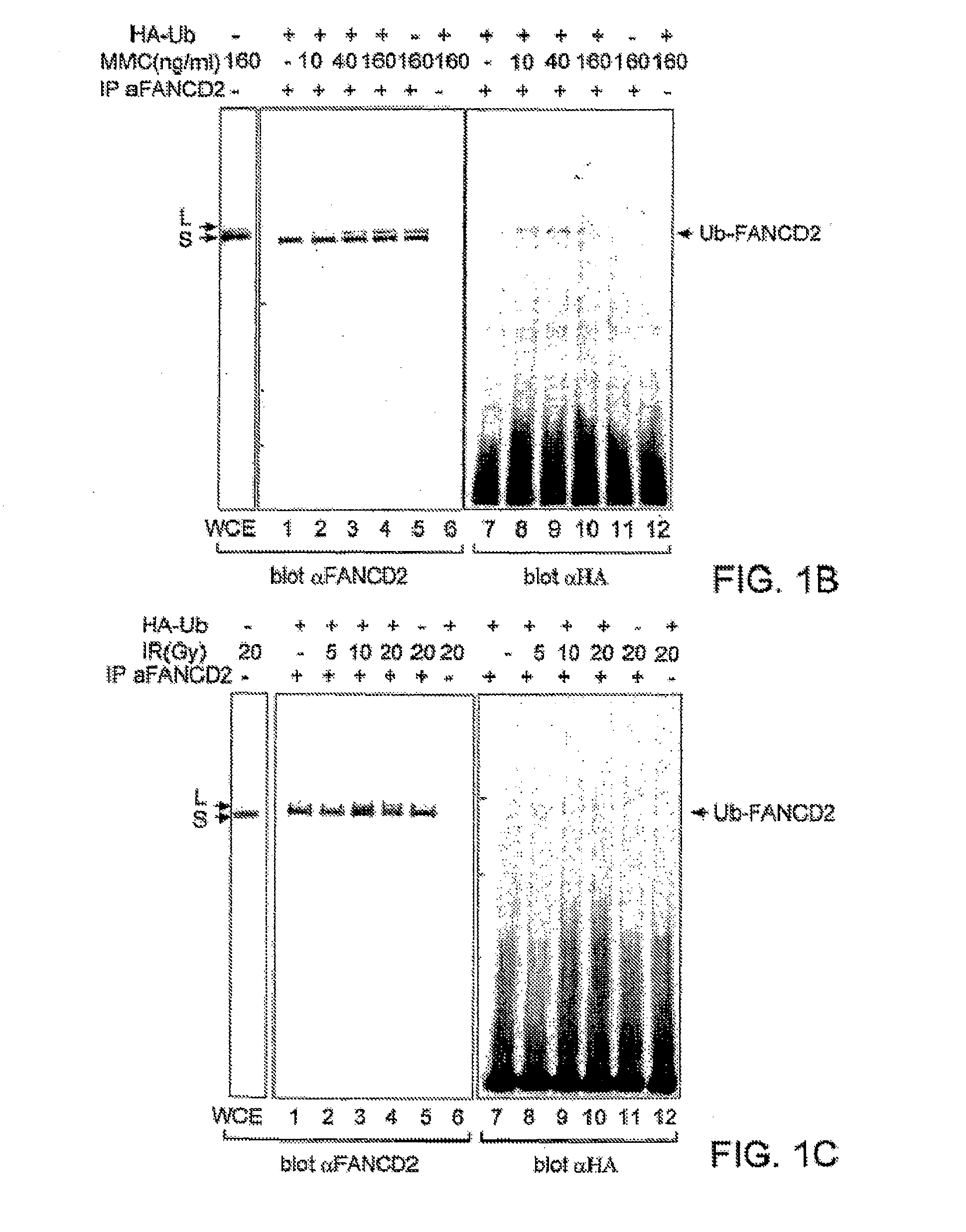
[0255]The high molecular weight isoform of FANCD2 could result from one or more mechanisms, including alternative splicing of the FANCD2 mRNA or post-translational modification(s) of the FANCD2 protein. Treatment with phosphatase did not convert FANCD2-L to FANCD2-S, demonstrating that phosphorylation alone does not account for the observed difference in their molecular mass.
[0256]In order to identify other possible post-translational modifications of FANCD2, we initially sought cellular conditions which regulate the conversion of FANCD2-S to FANCD2-L (FIGS. 1B, C). Since FA cells are sensitive to MMC and IR, we reasoned that these agents might regulate the conversion of FANCD2-S to FANCD2-L in normal cells. Interestingly, HeLa cells treated with MMC (FIG. 1B, lanes 1-6) or IR (FIG. 1C, lanes 1-6) demonstrated a dose-dependent increase in the expression of the FANCD2-L isoform.
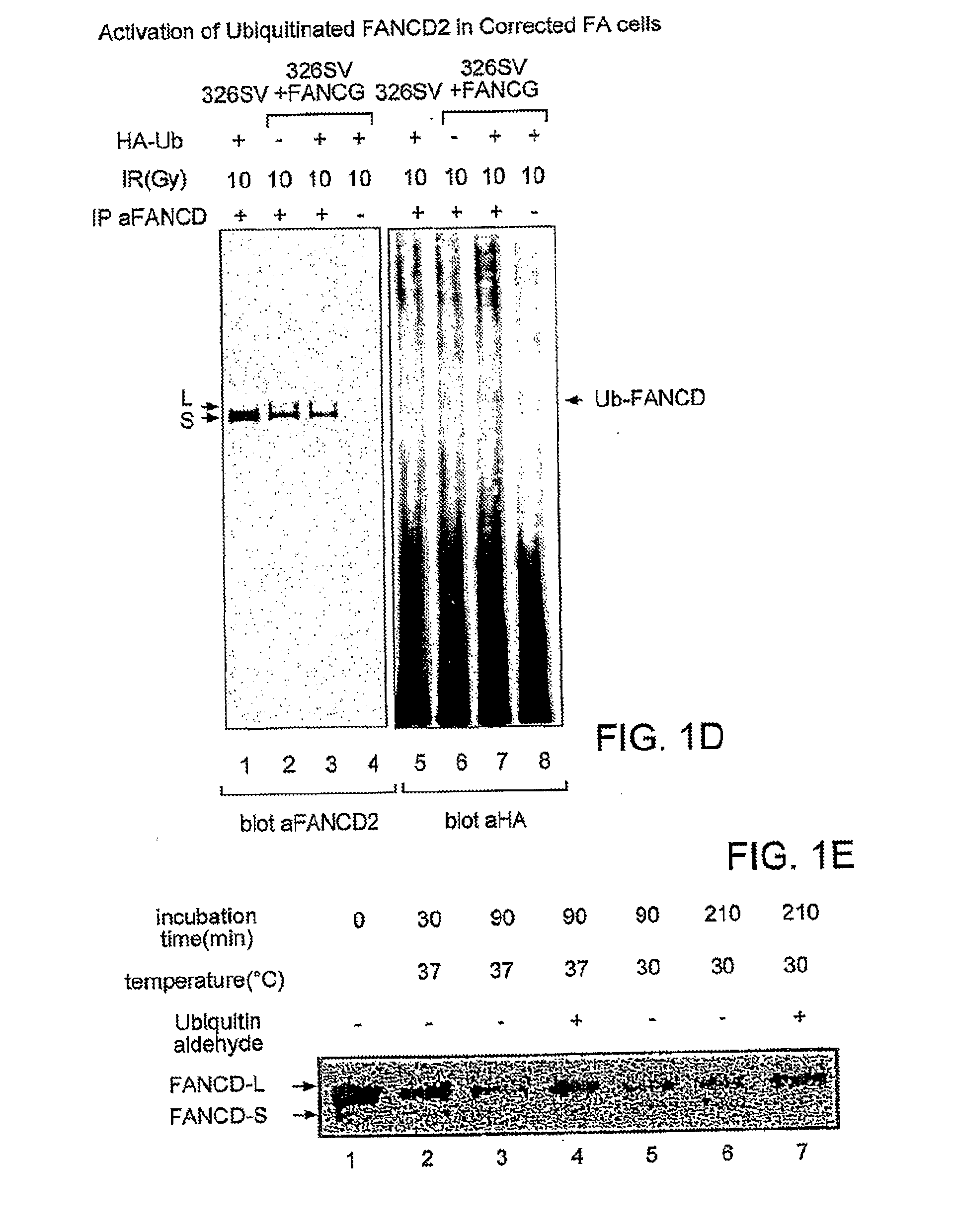
[0257]To determine whether FANCD2-L ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com