Core-Excited Nanoparticles and Methods of Their Use in the Diagnosis and Treatment of Disease
a nanoparticle and core-excited technology, applied in the field of core-excited nanoparticles, can solve the problems of limited practical clinical value of nir for most cancers, limited light emission efficiency, and large amount of heat generation, and achieves minimal side effects, low cost, and practical
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Targeting Breast Cancer
[0150]The following CENT treatment approach is based on a core-shell nanoparticle designed on the basis of X-ray excited persistent luminescence and the maximum safe dose of X-ray radiation the patient may tolerate. As a reference point for the therapeutic dosages of X-ray radiation needed, the patient is 180 cm in height and 80 Kg in weight.
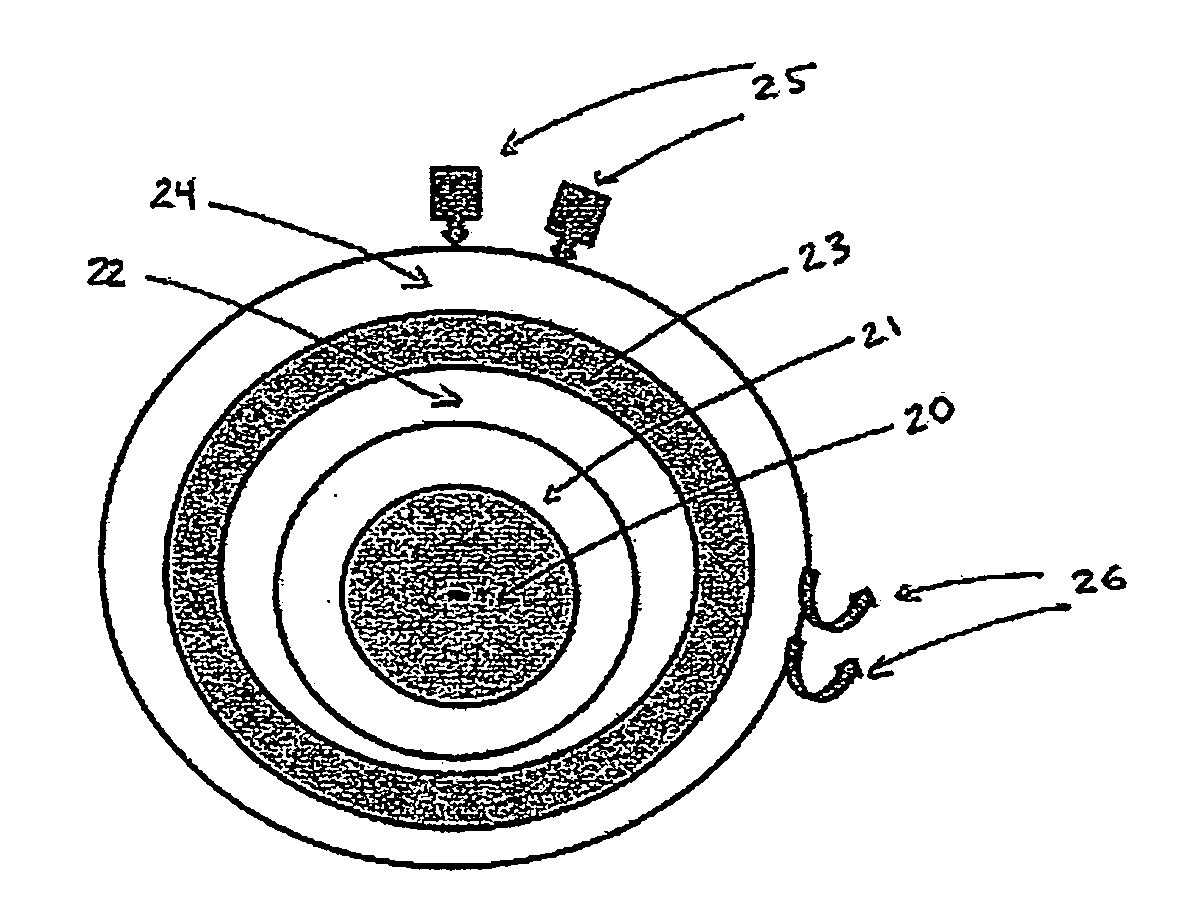
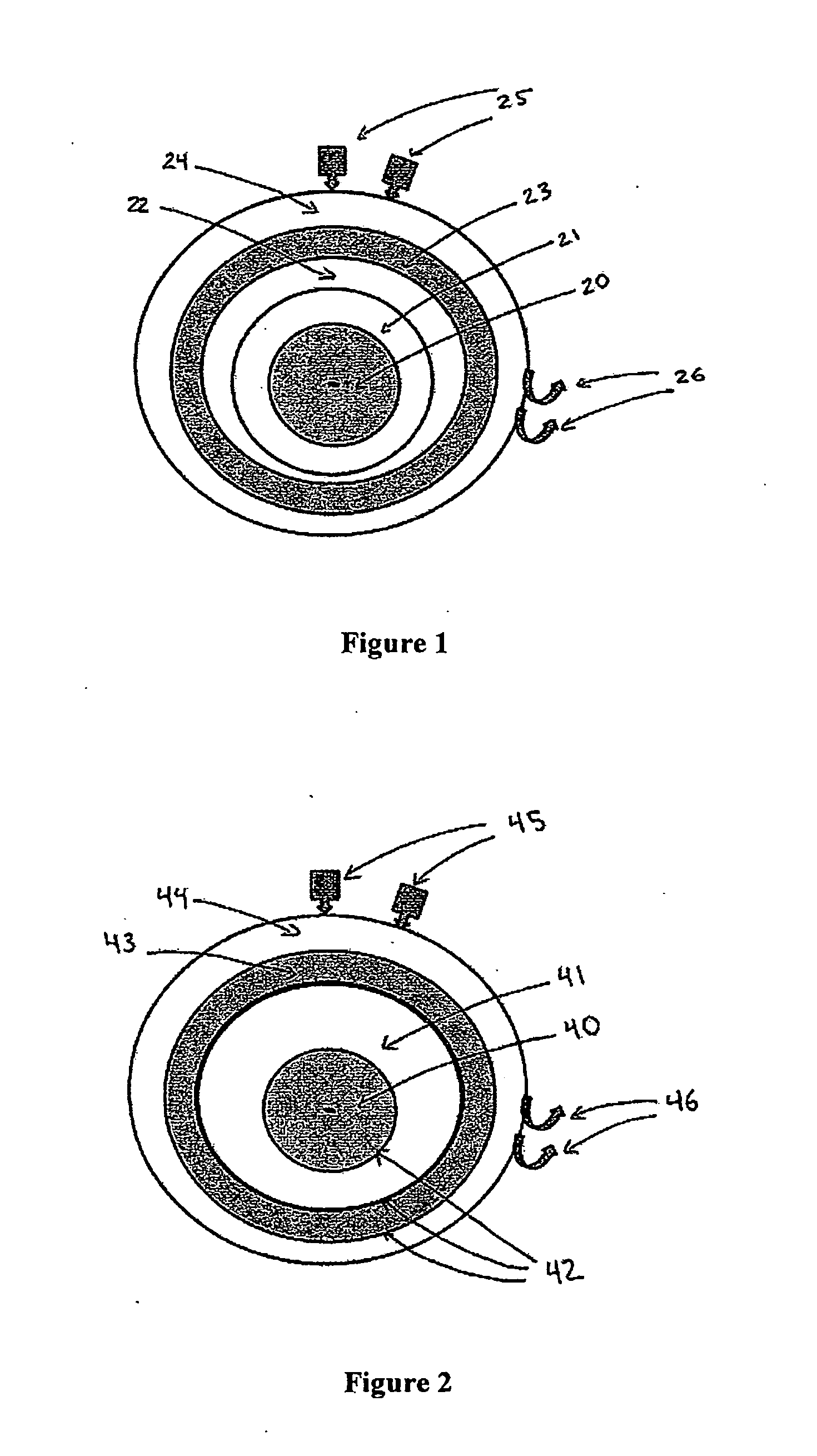
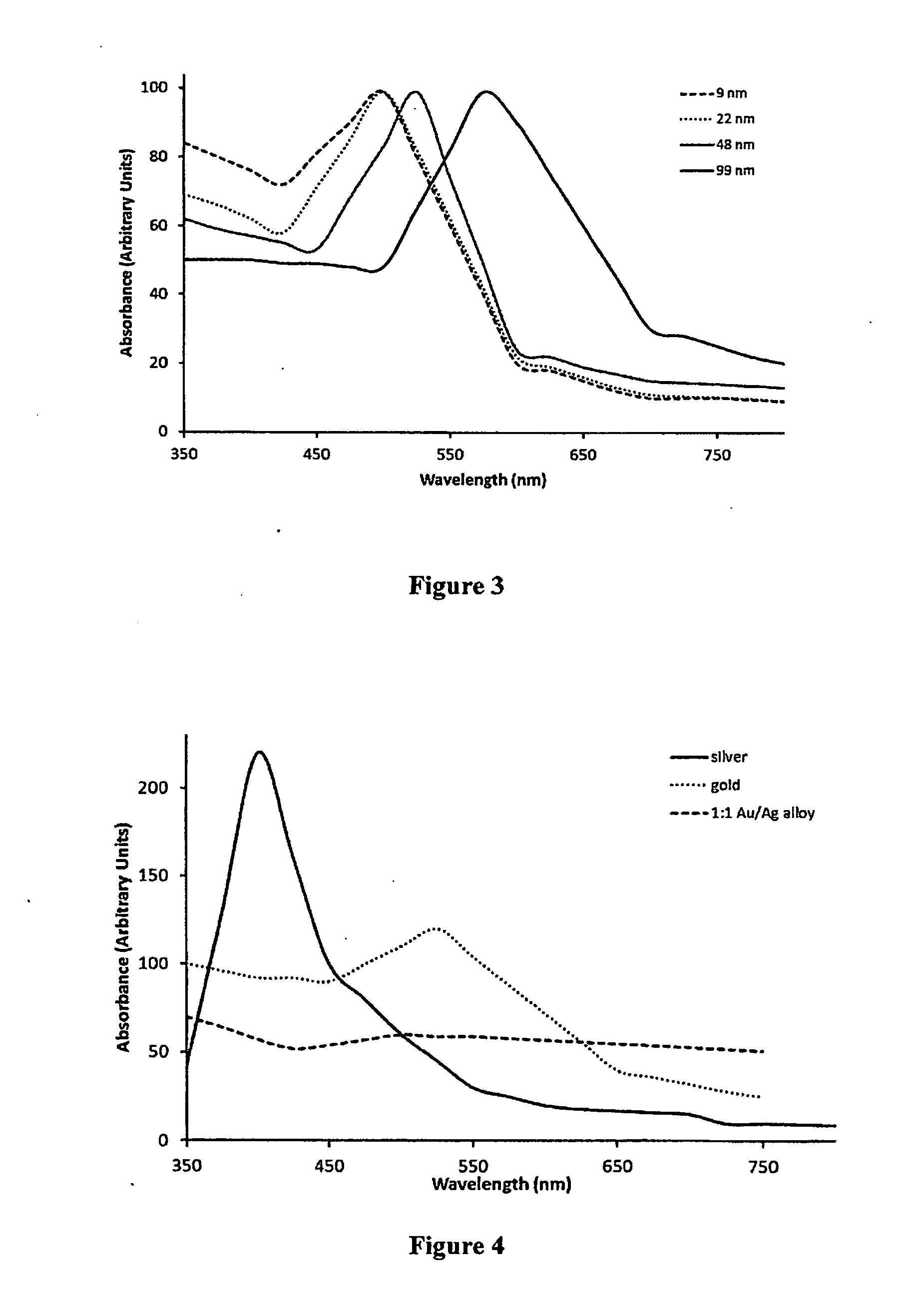
[0151]First, nanoparticles of SrAl2O4:Eu:Dy of approximately 60 nm in diameter, prepared by solid state reaction methods, are used as the core in a core-shell nanoparticle material design. The X-ray luminescence spectrum of this material has a maximum at approximately 510 nm, similar to the non-doped SrAl2O4 material, as shown in FIG. 7. A nanoshell of gold is grown over the nanocore material. Gold nanoparticles have the absorbance spectrum typical of that shown in FIGS. 3 and 4; additional Mie theory calculations allow design for maximum spectral overlap (FRET) between core and shell. The nanoparticle is then coated with ...
example 2
Treatment of Colon Cancer
[0153]The following CENT treatment approach is based on a core-shell nanoparticle designed on the basis of X-ray excited persistent luminescence and the maximum safe dose of computed tomography (CT) radiation the patient may tolerate. As a reference point for the therapeutic dosages of X-ray radiation needed, the patient is 180 cm in height and 80 Kg in weight.
[0154]First, nanoparticles of BaFBr:Eu2+, Mn2+ of 20 nm in diameter are prepared as the core material as outlined in Chen and Zhang, J. Nanoscience and Nanotechnology 6, 1159-1166, 2006. The X-ray luminescence spectrum of this material has a maximum at approximately 400 nm, as shown in FIG. 6A. A nanoshell of silver then is grown over the nanocore material. Silver nanoparticles have the absorbance spectrum typical of that shown in FIG. 4; additional Mie theory calculations allow design for maximum spectral overlap (FRET) between core and shell. The nanoparticle is then coated with polyethylene glycol (...
example 3
Treatment of Colon Cancer Using Nanoparticles Containing a Radionucleotide in the Nanoparticle Core
[0159]The following CENT treatment approach is based on a core-shell nanoparticle designed to a typical geometry of a 100-nm diameter core of Pd-103 and a 20-nm thick encasing layer of LaPO4:Ce, Tb and a 20-nm thick shell of gold. The radionuclide Pd-103 is an Auger electron emitter, with a 17-day half-life, that excites the Ce- and Tb-doped LaPO4 encasing layer to emit 543-nm green light, with a quantum yield of approximately 80%. The green light is absorbed by the gold shell (See FIG. 3) and heats the nanoparticle region that contains the targeted elements of the treatment, which are colon cancer cells in blood, lymph and tissue (tumors). The maximum dose of these nanoparticles that can be safely infused is administered to the patient. As a reference point for the therapeutic dosages, the patient is 180 cm in height and 80 Kg in weight.
[0160]First, nanoparticles of Pd-103 of 100-nm i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com