Multispecific antigen-binding molecules and uses thereof
a multi-specific, antigen-binding technology, applied in the field of therapeutic proteins, can solve the problems of unintended side effects, high technical complexity of strategies, and interference with the normal biological activity of antigens, and achieve the effects of improving the therapeutic effect, and improving the therapeutic
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Use of a Multispecific Antigen-Binding Molecule to Induce Degradation of a Cell Surface Receptor Via Linkage with an Internalizing Effector Protein
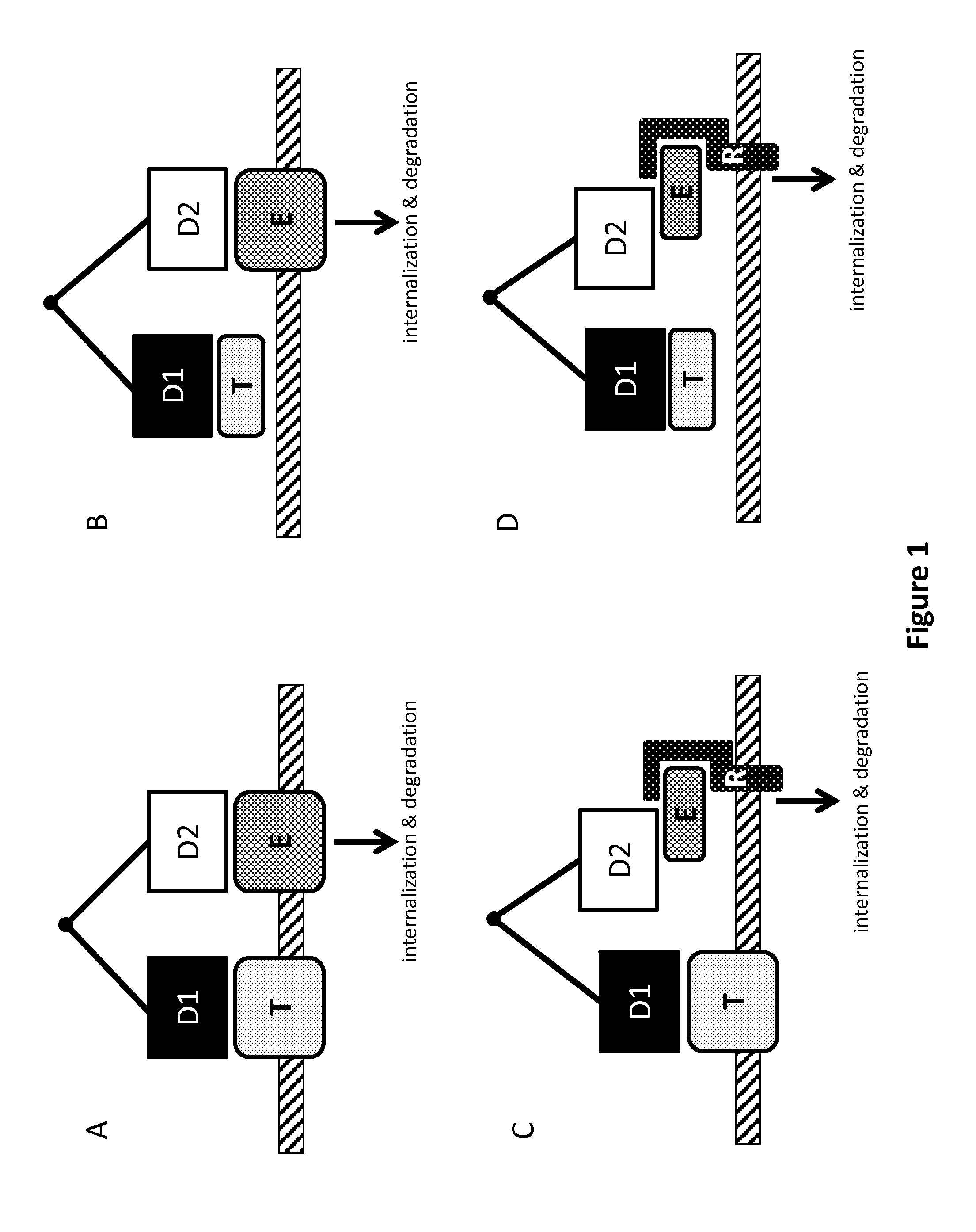
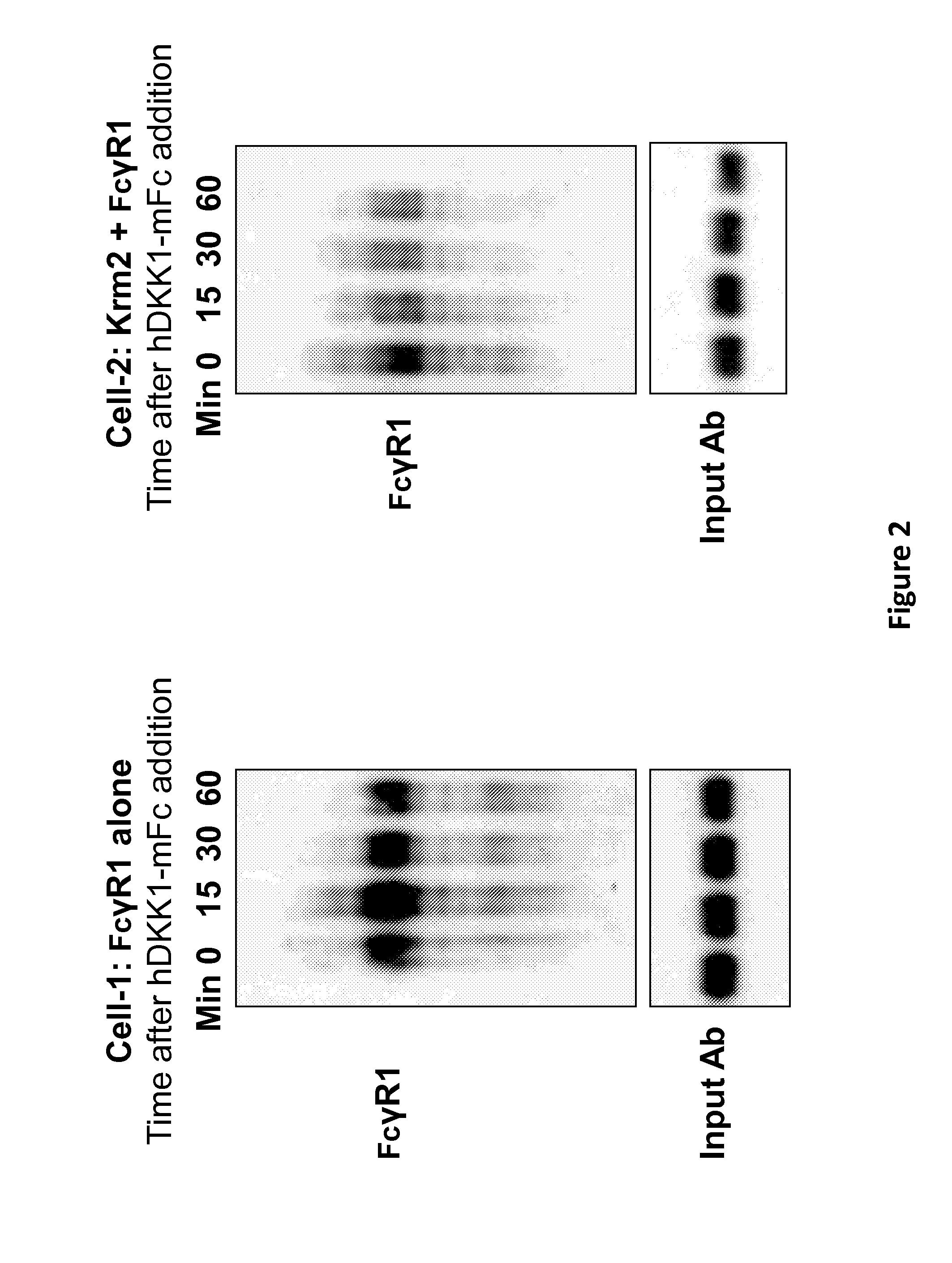
[0070]As an initial proof-of-concept experiment, a multispecific antigen-binding molecule was created which is capable of binding (a) an internalizing effector molecule and (b) a cell surface receptor target molecule. In this Example, the internalizing effector protein is Kremen-2 (Krm2), and the cell surface receptor target molecule is an Fc receptor (FcγR1 [Fc-gamma-R1]).
[0071]Kremen molecules (Krm1 and Krm2) are cell-surface proteins known to mediate WNT signaling by directing the internalization and degradation of the WNT pathway signaling molecules LRP5 and LRP6. Internalization of LRP5 / 6 is accomplished via the soluble interacting protein DKK1. In particular, DKK1 links Kremen to LRP5 / 6 on the cell surface, and because of this linkage, the internalization of Kremen drives the internalization and degradation of LRP5 and LRP6. (See Li...
example 2
IL-4R Activity is Attenuated Using a Multispecific Antigen-Binding Molecule with Specificity for IL-4R and CD63
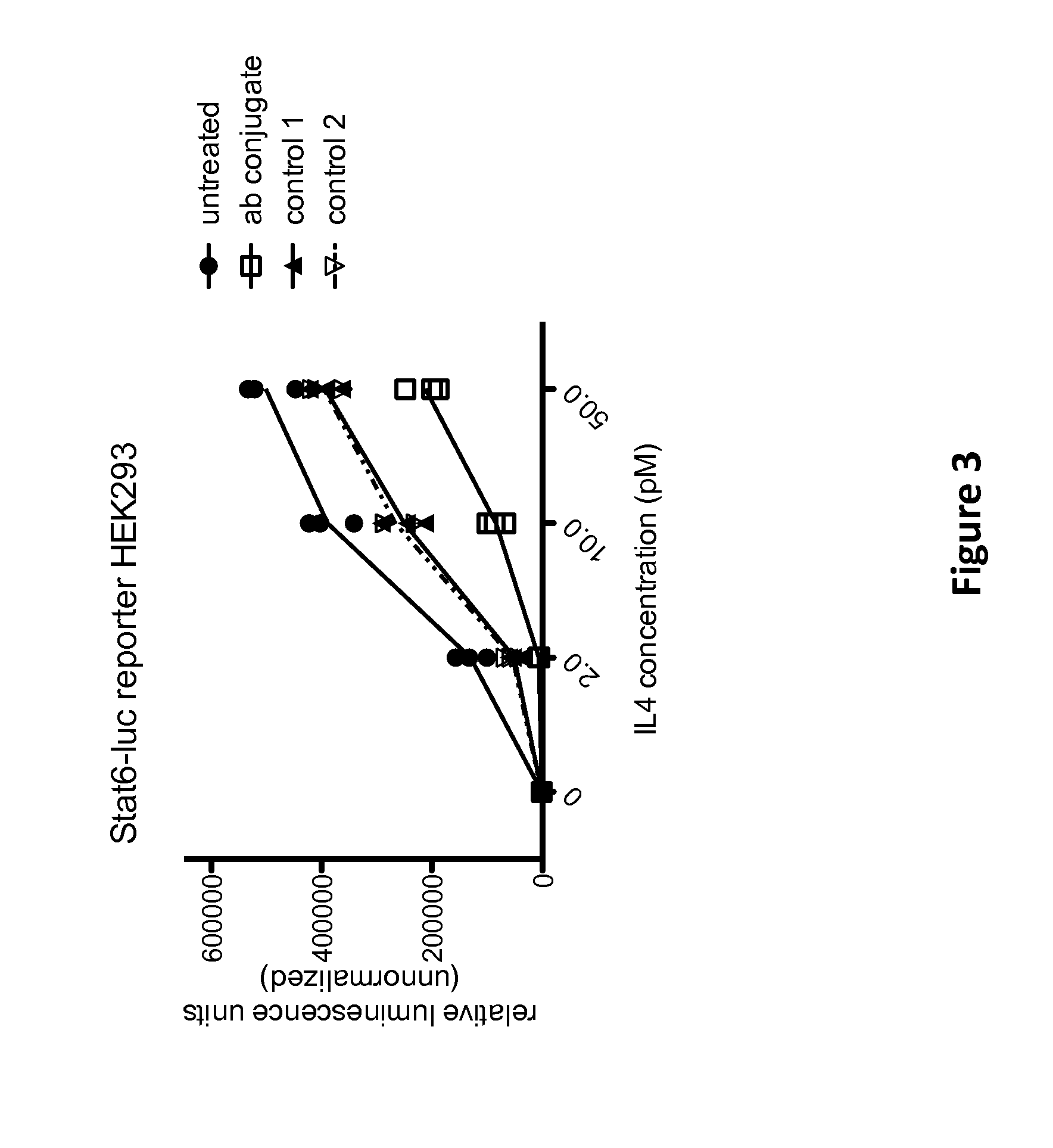
[0077]In a further set of proof-of-concept experiments, a multispecific antigen-binding molecule was constructed which is capable of simultaneously binding a cell surface-expressed target molecule (i.e., IL-4R) and a cell surface-expressed internalizing effector protein (i.e., CD63). The purpose of these experiments was to determine whether IL-4R activity on a cell can be attenuated to a greater extent by physically linking IL-4R to an effector molecule that is internalized and targeted for degradation within the lysosome (in this case, CD63). In other words, this Example was designed to test whether the normal internalization and degradation of CD63 could be used to force the internalization and degradative rerouting of IL-4R within a cell.
[0078]First, a multispecific antigen-binding molecule was constructed that is able to bind both IL-4R and CD63. Specifically, a strepta...
example 3
An Anti-IL-4R×Anti-CD63 Bispecific Antibody Attenuates IL-4R Activity in a CD63-Dependent Manner
[0086]The experiments of Example 2, herein, show that an anti-IL-4R / anti-CD63 multispecific molecule inhibits IL-4-mediated signaling in a CD63-dependent manner. In those experiments, the multispecific antigen-binding molecule consisted of two separate monoclonal antibodies (anti-IL-4R and anti-CD63) that were connected via a biotin-streptavidin linkage. To confirm that the results observed with that proof-of-concept multispecific antigen-binding molecule are generalizable to other multispecific antigen-binding molecule formats, a true bispecific antibody was constructed.
[0087]Standard bispecific antibody technology was used to construct a bispecific antibody consisting of a first arm specific for IL-4R and a second arm specific for CD63. The IL-4R-specific arm contained an anti-IL-4R heavy chain paired with a CD63-specific light chain. The CD63-specific light chain was paired with the IL...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Solubility (mass) | aaaaa | aaaaa |
| Affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com