Method for identifying immunoreactive peptides
a technology of immunoreactive peptides and immunoreactive peptides, which is applied in the field of immunoreactive peptide identification methods, can solve the problems of large number of candidate peptide identification, large number of culture techniques, and restriction of the frequency of pre-existing t-cells, and achieve the effect of simple and selective identification of immunoreactive peptides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Patient Samples
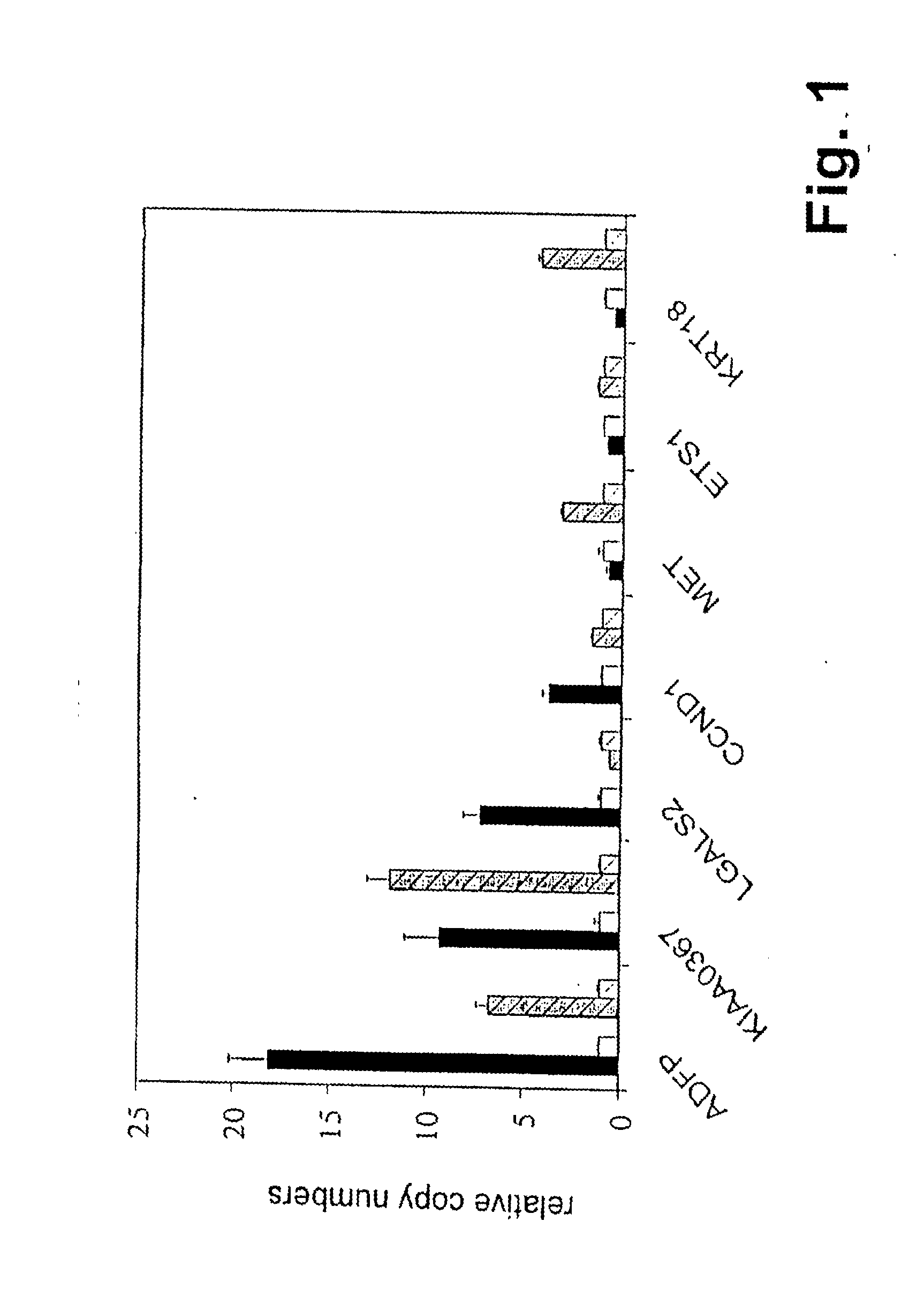
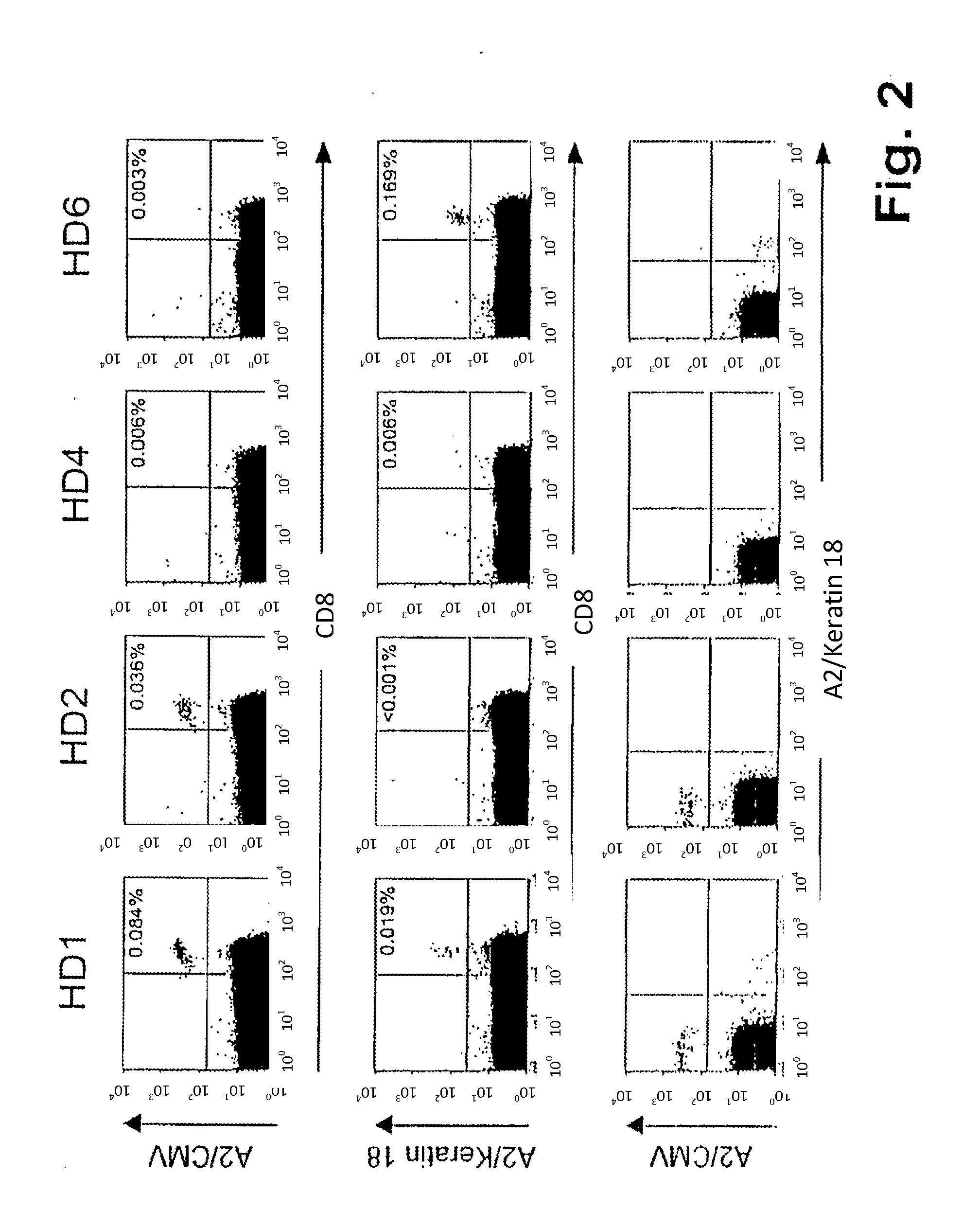
[0069]Samples of patients having histologically confirmed renal cell carcinoma were obtained from the department of urology, University of Tubingen. Both patients had not received preoperative therapy. Patient No. 1 (in the following designated RCC01) had the following HLA-typing: HLA-A*02 A68 B*18 B*44; patient No 2 (in the following designated RCCI3) HLA-A*02 A*24 B*07 B*40.
Isolation of MHC Class 1-Bound Peptides
[0070]Shock-frozen tumor samples were processed as described in Schirle, M. et al., Identification of tumor-associated MHC-class I ligands by a novel T-cell-independent approach, 2000, European Journal of Immunology, 30: 2216-2225. Peptides were isolated according to standard protocols using monoclonal antibody W6 / 32 being specific for HLA class I or monoclonal antibody BB7.2 being specific for HLA-A2. Production and utilization of these antibodies is described by Barnstable, C. J. et al., Production of monoclonal antibodies to group A erythrocytes, HLA and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volumes | aaaaa | aaaaa |
| flow rate | aaaaa | aaaaa |
| flow rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com