Therapeutic vaccination against active tuberculosis
a technology of active tuberculosis and therapeutic vaccination, applied in the field of biotechnology and health care, can solve the problems of not being able to recognize and kill intracellular tb organisms, and achieve the effect of reducing the burden of mtb
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Clinical Trial with Ad35-Based TB Vaccine (Ad35.TB-S) in Human Subjects
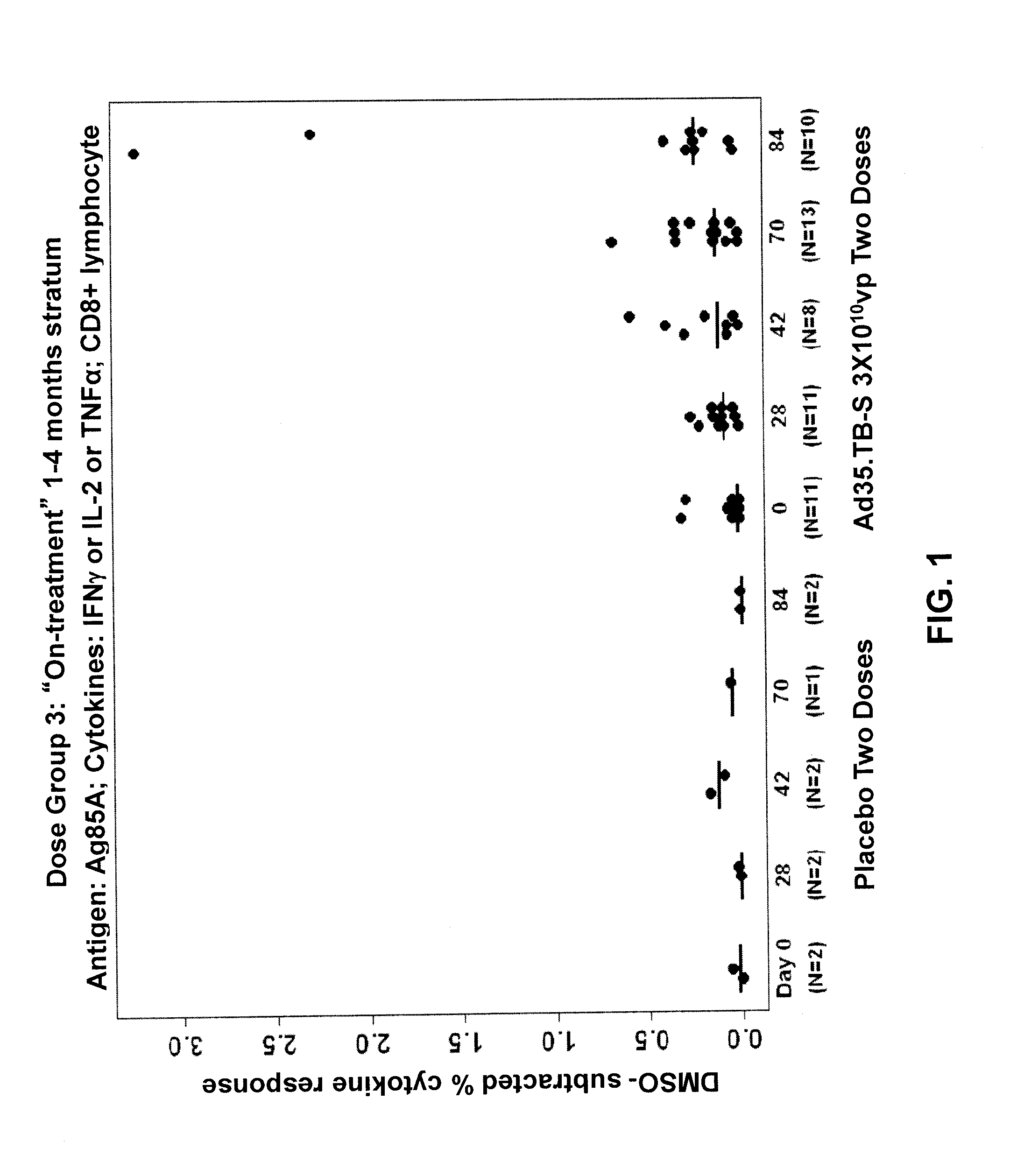
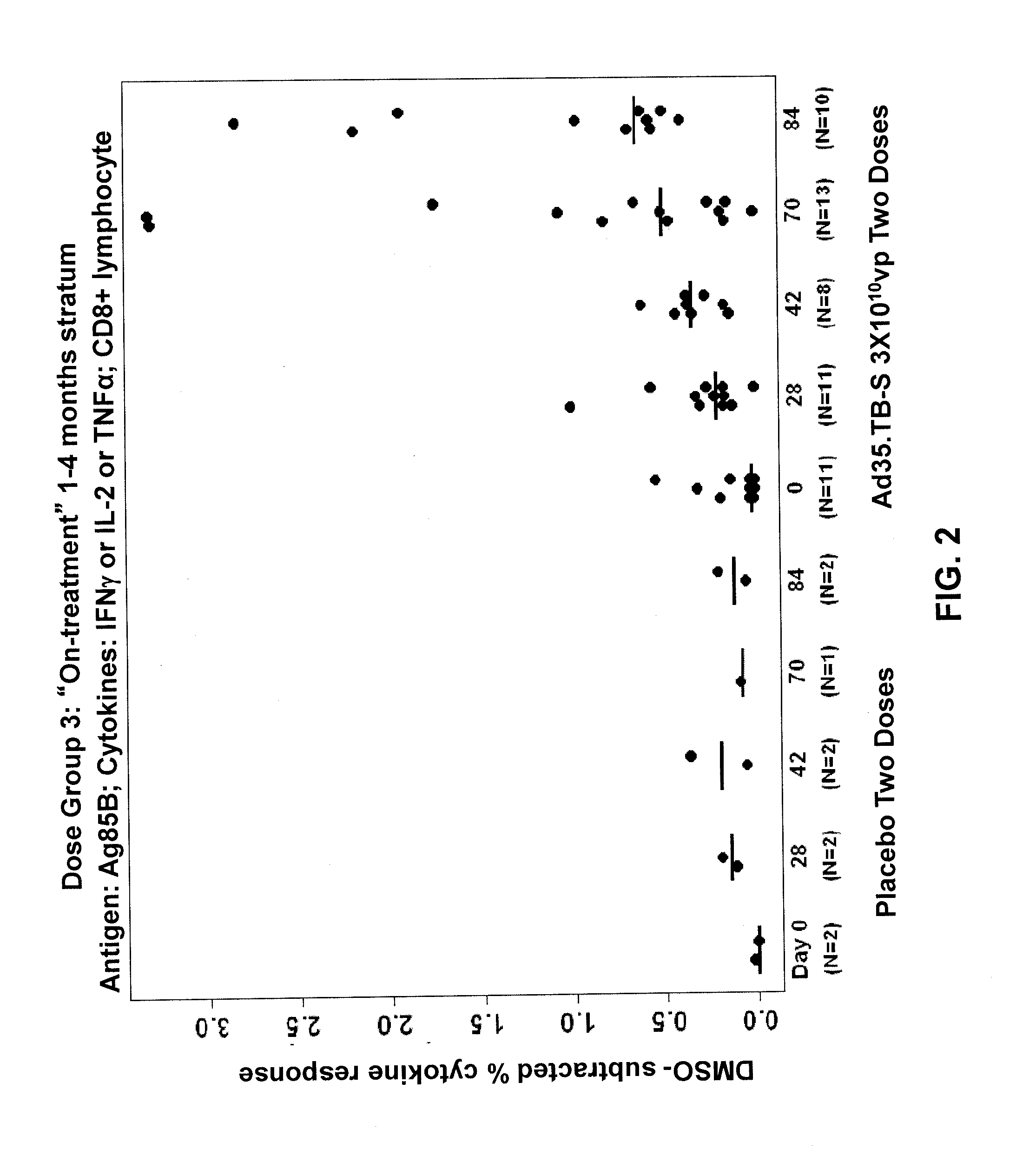
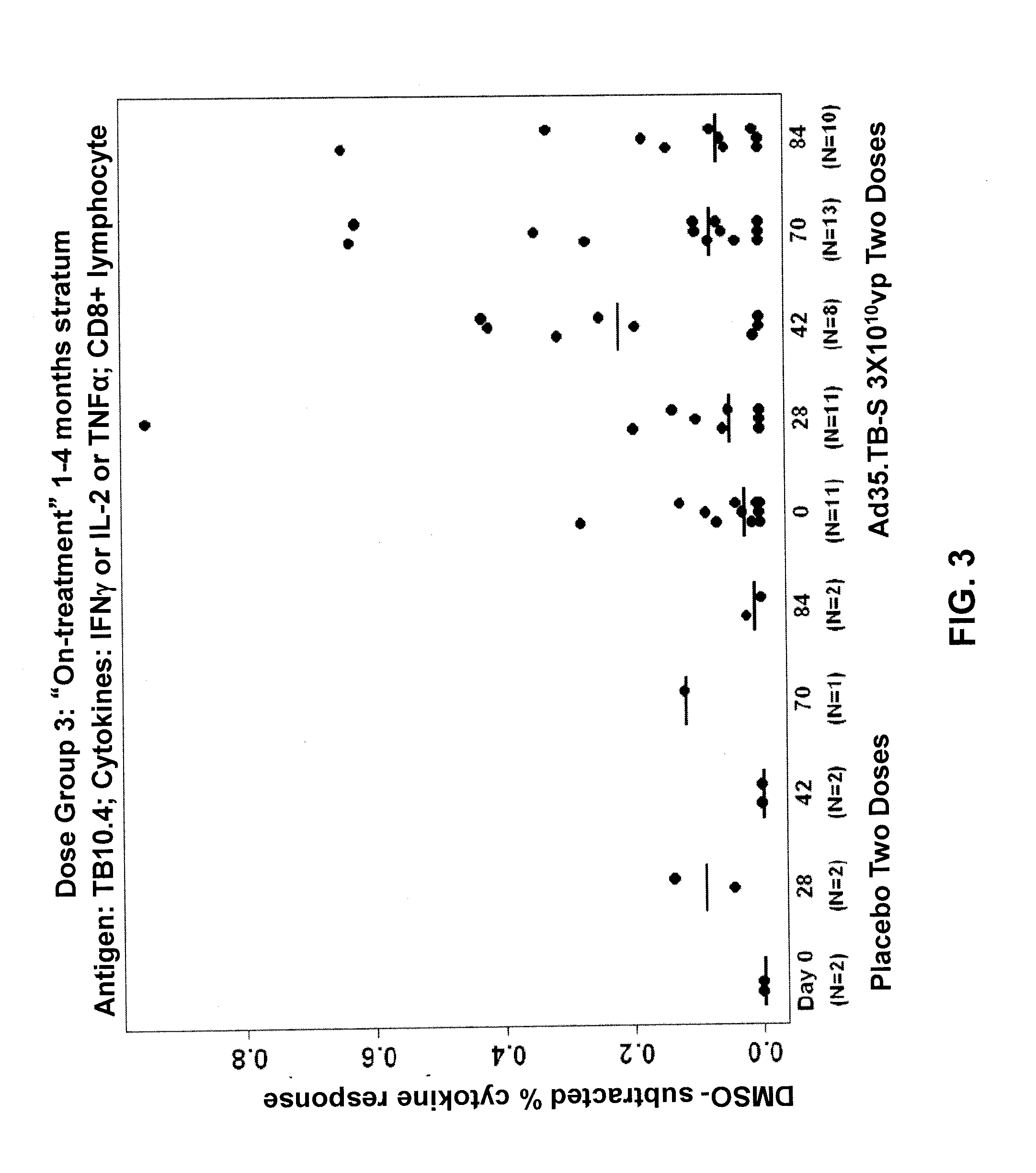
[0092]A randomized, double-blinded, placebo-controlled clinical trial on human subjects was performed to evaluate the safety and immunogenicity of Ad35.TB-S (Havenga et al., 2006; Rado{hacek over (s)}ević et al., 2007; WO 2006 / 053871) in individuals with prior or current tuberculosis. This trial was conducted to ensure that the vaccine did not elicit severe adverse reactions, such as Koch's phenomenon, in subjects with previously unrecognized or active tuberculosis. By ensuring the safety of the vaccine in patients clinically documented to have tuberculosis, the vaccine could then be administered widely to larger populations in future studies without the need for extensive TB testing among subjects.
[0093]The study was designed as a dose escalation study where the vaccine dosage was increased in successive patient groups. Patients were enrolled sequentially, with the patients receiving the lowest dosage of vaccine...
example 2
Proof of Concept Study to Show the Treatment-Shortening Effect of the Ad35.TB-S Vaccine on Tuberculosis Drug Therapy in Mice
[0099]Here, a method to show the TB chemotherapy-shortening effect of Ad35.TB-S is described. The method utilizes an established mouse model for TB drug chemotherapy, which is adapted for experimentation with the Ad35.TB-S vaccine.
[0100]Treatment of humans with Isoniazid, Rifampicin and Pyrazinamide in the first two months followed by Rifampicin and Isoniazid for the remaining four months of therapy results in a 1% to 2% chance of disease relapse (Neurmberger, 2008; Fox et al., 1999). In mice, similar therapy results in a 0% to 10% chance of relapse (Neurmberger, 2008). In a recent study, BALB / C mice exhibited a 0% relapse proportion when treated with this six-month standard regimen, which rose to 90% when treatment was shortened to four months (Williams et al., 2009). In that study, “relapse” was defined as isolation of 1 or greater CFU after plating the entir...
example 3
The Effect of Therapeutic Vaccination by Heterologous Boosting of Ad35.TB-S with Ad26.TB-S Vaccines in a Mouse Model of TB Therapy
[0107]The first heterologous prime-boost vaccine was trialed in humans by the group of Adrian Hill at Oxford (Schneider et al., 1998) in a trial designed to study the immunogenicity of a prophylactic vaccine against malaria. It was then observed that priming and boosting with different vectors carrying the same antigens resulted in a markedly enhanced immune response, which was due to the proliferation of memory T cells.
[0108]To test if priming with Ad35 and boosting with Ad26 would markedly result in enhanced immunogenicity as well as a decrease in relapse proportions upon shortening of TB therapy, two additional arms were added to the study shown in Example 2 (FIG. 6). In one arm, an additional vaccination of Ad26.TB-S containing the same antigens as the Ad35.TB-S was given at the end of the four-month therapy. Should the Ad26.TB-S boost enhance the imm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com