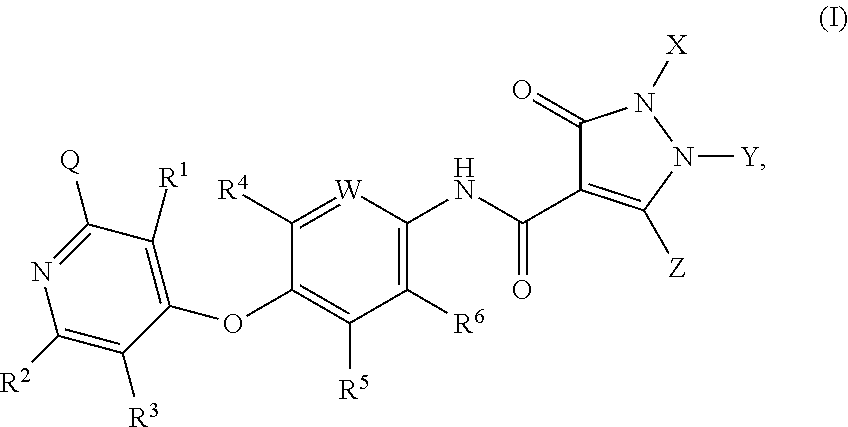
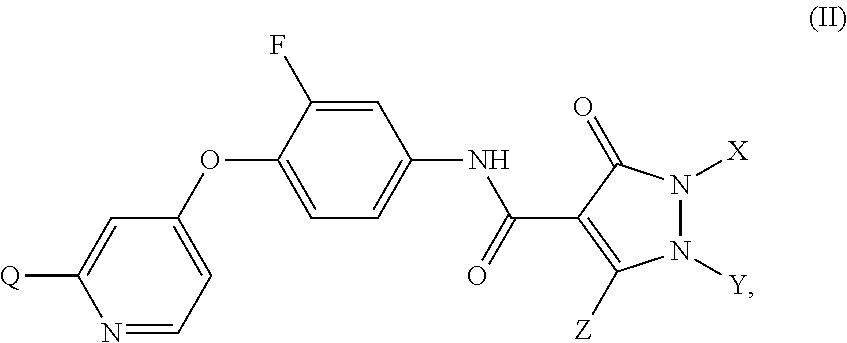
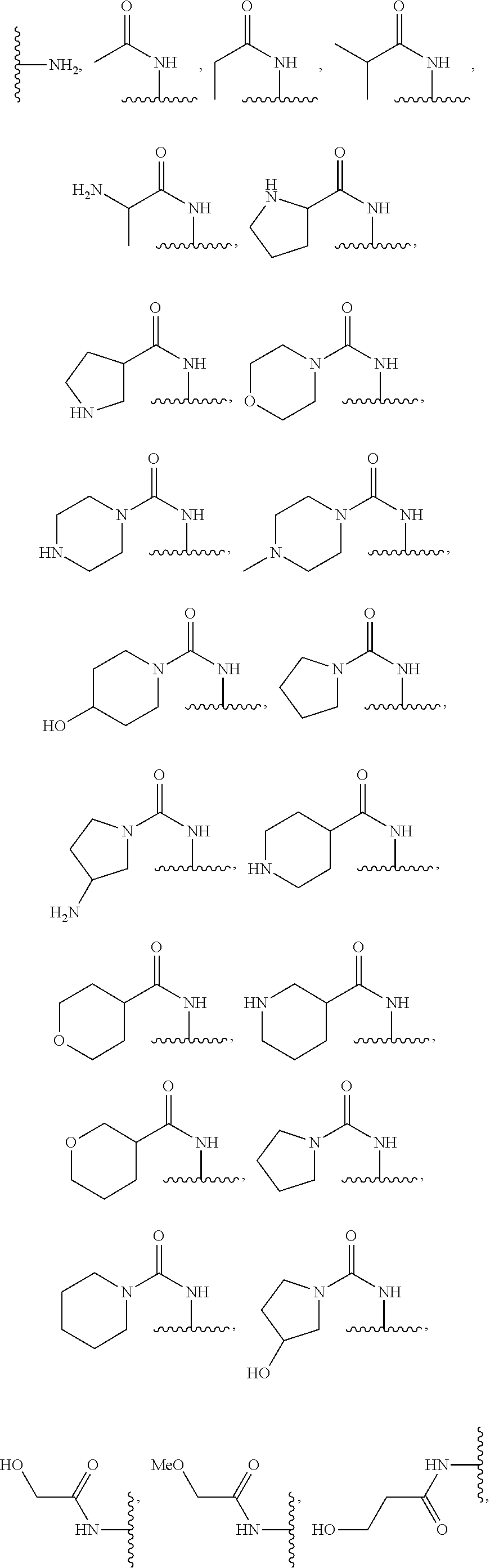
Substituted pyrazolone compounds and methods of use
a technology of pyrazolone and compounds, applied in the field of new substituted pyrazolone compounds, can solve the problems of reducing the chance of resistance developmen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
N-(4-((2-aminopyridin-4-yl)oxy)phenyl)-1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazole-4-carboxamide
[0177]
Step 1) 4-aminophenol
[0178]To a mixture of 4-nitrophenol (7 g, 50.3 mmol) and HCOOK (1.8 g, 21.48 mmol) in THF (210 mL) and H2O (70 mL) was added Pd / C (110 mg, 10% Pd content, 53%˜55% water content). The reaction was stirred at 50° C. for 24 hours, and then concentrated in vacuo. The residue was diluted with DCM (100 mL) and filtered through a CELITE® pad. The filtrate was concentrated in vacuo to give the title compound as a pale orange solid (3.28 g, 60%).
[0179]MS (ESI, pos. ion) m / z: 110.1 [M+H]+.
Step 2) 4-(4-aminophenoxy)pyridin-2-amine
[0180]To a mixture of 4-aminophenol (218 mg, 2 mmol) and 4-chloropyridin-2-amine (256 mg, 2 mmol) in DMSO (2.5 mL) was added NaOCH3 (216 mg, 4 mmol). The reaction was microwaved at 180° C. for 40 minutes, then cooled down to rt and quenched with water (10 mL). The mixture was extracted with EtOAc (10 mL×3). The combined organic phases were...
example 2
N-(4-((2-amino-3-chloropyridin-4-yl)oxy)phenyl)-1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazole-4-carboxamide
[0186]
Step 1) N-(4-hydroxyphenyl)-1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazole-4-carboxamide
[0187]To a mixture of 4-aminophenol (1.09 g, 10 mmol) and 1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazole-4-carboxylic acid (2.37 g, 10.2 mmol) in DCM (30 mL) was added EDCI (2.3 g, 12 mmol) and HOAT (0.27 g, 2 mmol). The mixture was stirred at 46° C. for 4 hours, then cooled to rt and diluted with EtOAc (10 mL) and water (10 mL). The mixture was stirred at rt for 1 hour, then filtered to give the title compound as a white solid (1.7 g, 52.5%).
[0188]MS (ESI, pos. ion) m / z: 324.1 [M+H]+;
[0189]1H NMR (400 MHz, DMSO-d6): δ (ppm) 2.68 (s, 3H), 3.32 (s, 3H), 6.72 (d, J=8.8 Hz, 2H), 7.36-7.42 (m, 4H), 7.49 (t, J=7.4 Hz, 1H), 7.57 (t, J=7.6 Hz, 2H), 9.21 (s, 1H), 10.46 (s, 1H).
Step 2) 3,4-dichloropicolinamide
[0190]To a mixture of 2,2,6,6-tetramethylpiperidine (6.2 mL, 37.2 mmol...
example 3
N-(4-((2-amino-3-chloropyridin-4-yl)oxy)-3-fluorophenyl)-1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazole-4-carboxamide
[0199]
Step 1) 4-(4-amino-2-fluorophenoxy)-3-chloropicolinamide
[0200]To a solution of 4-amino-2-fluorophenol (254 mg, 2.0 mmol) in DMF (5 mL) was added t-BuOK (359 mg, 3.2 mmol). The mixture was stirred at rt for 30 minutes, then 3,4-dichloropicolinamide (420 mg, 2.2 mmol) was added, and the mixture was microwaved at 120° C. for 2 hours. The mixture was cooled to rt, quenched with 25 mL of water. The resulted solution was extracted with EtOAc (30 mL×3) and the combined organic phases were washed with brine (30 mL×3), dried over Na2SO4 and concentrated in vacuo. The residue was purified by a silica gel column chromatography (EtOAc / PE (v / v)=2 / 1) to give the title compound as a pale yellow solid (306 mg, 54.4%).
[0201]MS (ESI, pos. ion) m / z: 282.1 [M+H]+;
[0202]1H NMR (400 MHz, DMSO-d6): δ (ppm) 8.30 (d, J=5.6 Hz, 1H), 8.03 (s, 1H), 7.73 (s, 1H), 7.03 (t, J=9.0 Hz, 1H)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| v/v | aaaaa | aaaaa |
| v/v | aaaaa | aaaaa |
| v/v | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com