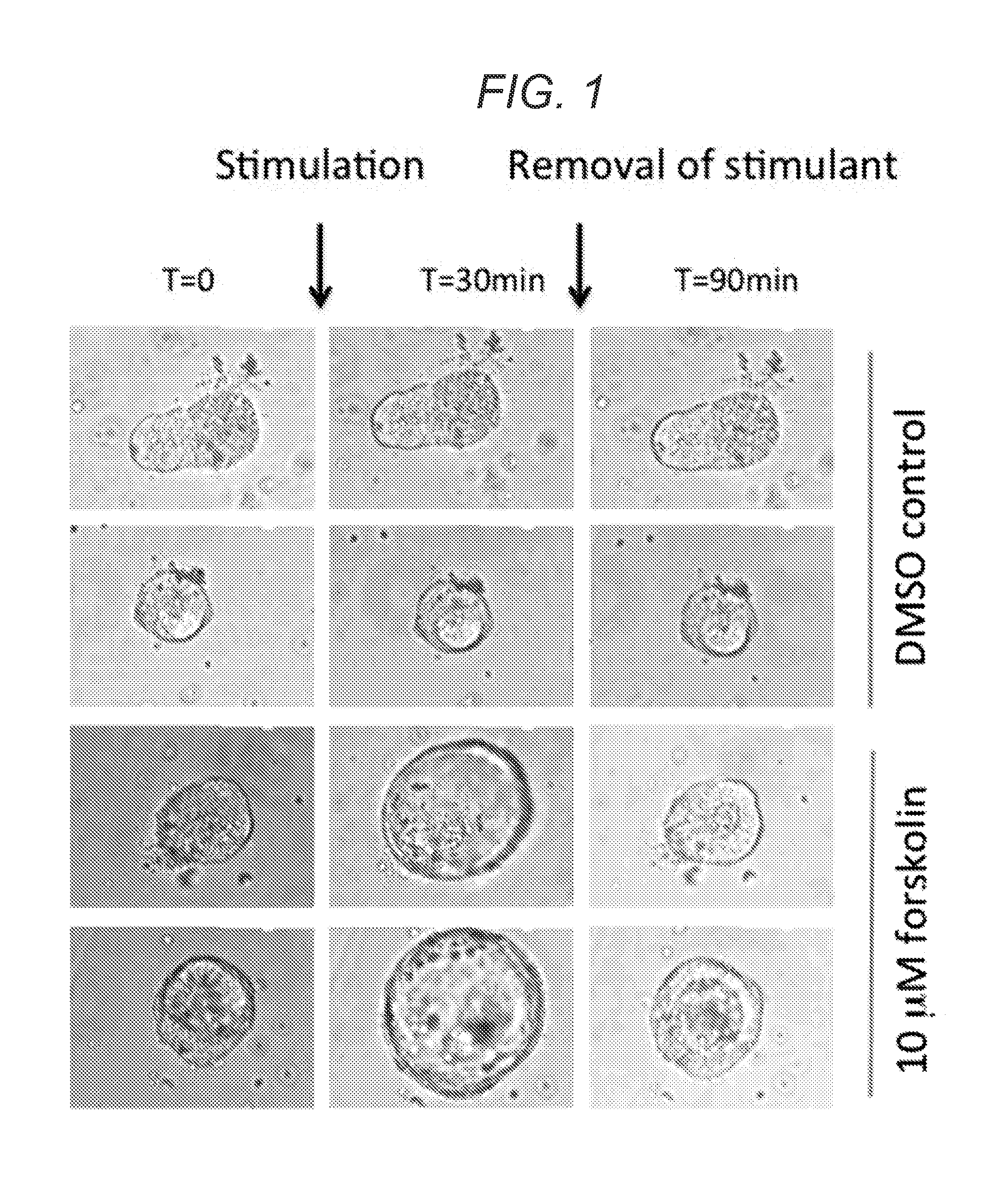
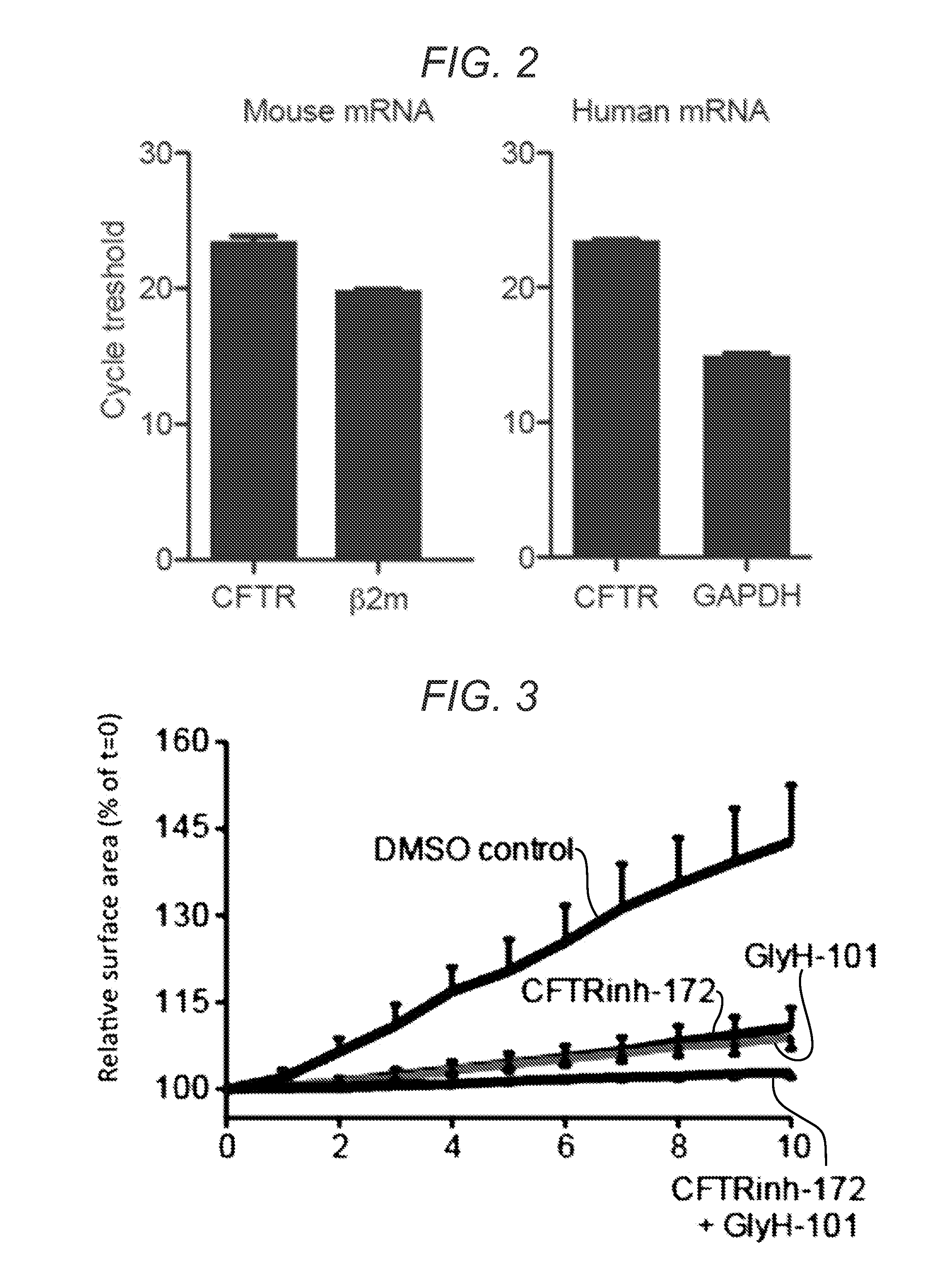
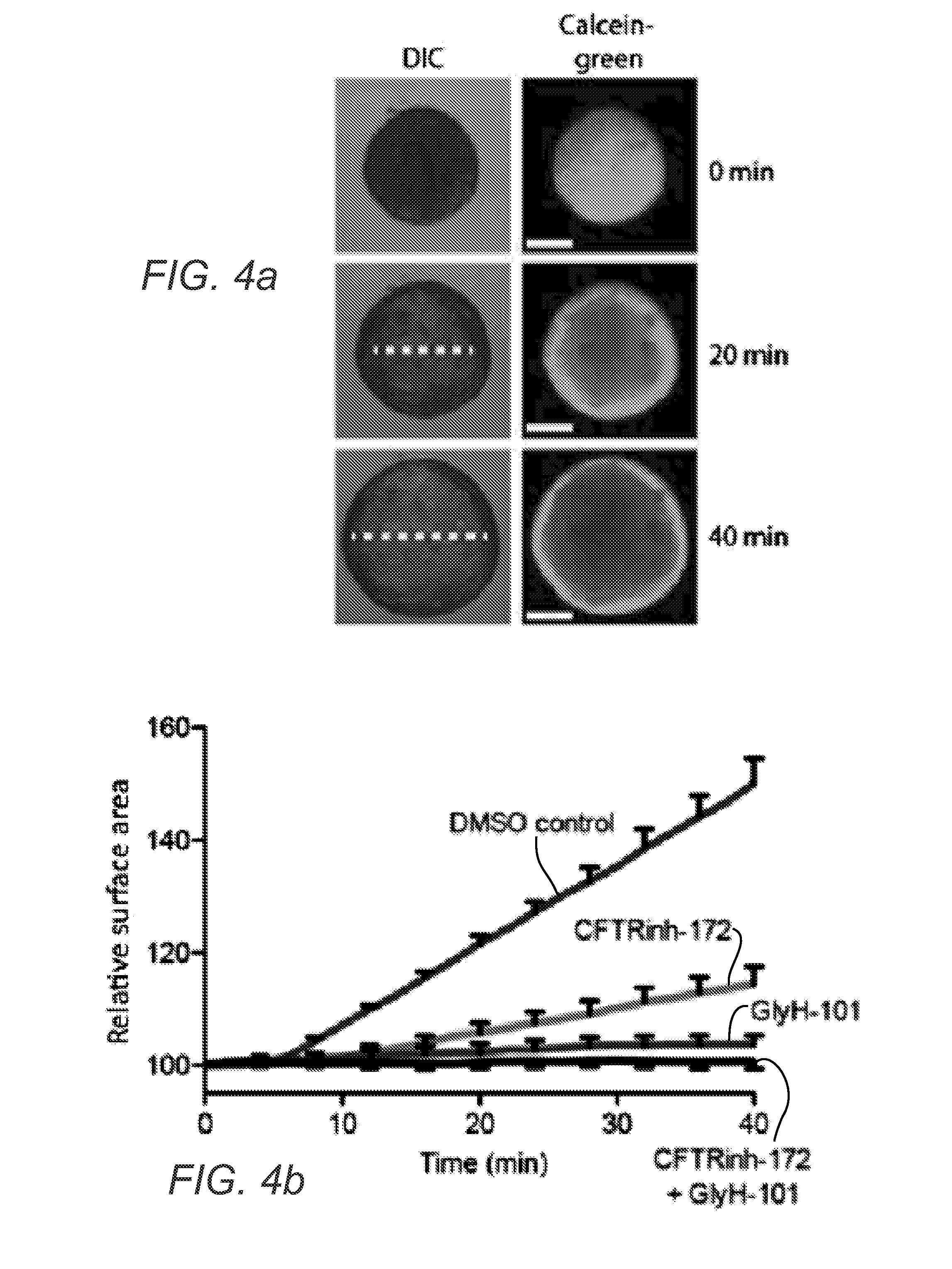
A rapid quantitative assay to measure cftr function in a primary intestinal culture model
a technology of cftr and culture model, applied in the field of fluid and electrolyte homeostasis assay, can solve the problems of cftr gene that has been found in cf patients, cftr gene accumulation in pulmonary and gastrointestinal tract, affecting the production of cftr, etc., and achieves rapid organoid swelling and minimal off-target activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
References for Example 1
[0174]Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche.[0175]Sato T, Vries R G, Snippert H J, van de Wetering M, Barker N, Stange D E, van Es J H, Abo A, Kujala P, Peters P J, Clevers H. Nature. 2009 May 14;459(7244):262-5
[0176]Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Sato T, Stange D E, Ferrante M, Vries R G, Van Es J H, Van den Brink S, Van Houdt W J, Pronk A, Van Gorp J, Siersema P D, Clevers H. Gastroenterology. 2011 Nov;141(5):1762-72.
Example 2
[0177]We have recently established conditions allowing long-term expansion of epithelial organoids from human intestine, recapitulating essential features of the in vivo tissue architecture. Here, we apply this technology to study primary intestinal organoids of patients that suffer from cystic fibrosis (CF), a disease caused by cystic fibrosis transmembrane conductance regulator (CFTR) gene mutations. F...
example 2
References for Example 2
[0212]1. Riordan, J. R. et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 245, 1066-1073 (1989).[0213]2. Rommens, J. M. et al. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science 245, 1059-1065 (1989).[0214]3. Kerem, B. et al. Identification of the cystic fibrosis gene: genetic analysis. Science 245, 1073-1080 (1989).[0215]4. Ratjen, F. & Döring, G. Cystic fibrosis. Lancet 361, 681-689 (2003).[0216]5. Cheng, S. H. et al. Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell 63, 827-834 (1990).[0217]6. Riordan, J. R. CFTR function and prospects for therapy. Annu. Rev. Biochem. 77, 701-726 (2008).[0218]7. Clancy, J. P. & Jain, M. Personalized medicine in cystic fibrosis: dawning of a new era. Am. J. Respir. Crit. Care Med. 186, 593-597 (2012).[0219]8. Ramsey, B. W. et al. A CFTR potentiator in patients with cystic f...
example 3
[0271]Cystic fibrosis transmembrane conductance regulator (CFTR) functions as anion channel, and is essential for fluid and electrolyte homeostasis at epithelial surfaces of many organs, including lung and intestine. The autosomal-recessive disorder cystic fibrosis (CF) is caused by mutations of the CFTR gene. CF disease is highly variable, and patients have a median life expectancy of approximately 40 years. Loss-of-function mutations cause altered ion and fluid transport that results in accumulation of viscous mucus in the pulmonary and gastrointestinal tract. This is associated with bacterial infections, aberrant inflammation and malnutrition. Over 1500 mutations have been described, but the most dominant mutation (˜67% of total mutant alleles worldwide) is a deletion of phenylalanine at position 508 (CFTR-delF508). This causes misfolding, ER-retention and early degradation of the CFTR protein which prevents function at the plasma membrane. Other mutations in the CFTR gene that h...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com