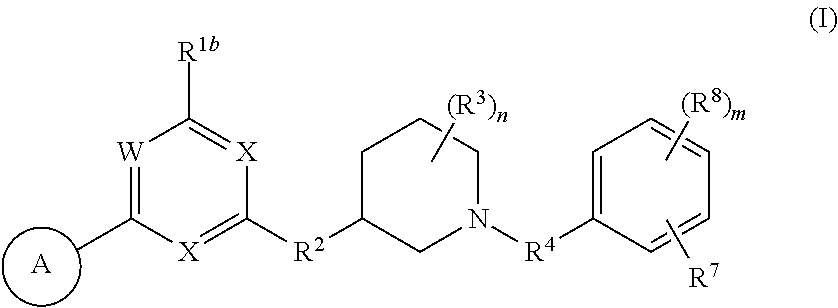
Heteroaromatic compounds useful for the treatment of prolferative diseases
a technology of heteroaromatic compounds and prolferative diseases, applied in the direction of drug compositions, organic chemistry, organic active ingredients, etc., can solve the problems of hampered discovery of selective inhibitors of cdk7 and anti-proliferative activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of (E)-N-(4-(3-(5-chloro-4-(1H-indol-3-yl)pyrimidin-2-ylamino)piperidine-1-carbonyl)phenyl)-4-(dimethylamino)but-2-enamide (Compound 100)
p-{[3-(Benzyloxycarbonylamino)-1-piperidyl]carbonyl}phenylamino 2,2-dimethylpropionate
[0170]
[0171]To a solution of 4-(tert-butoxycarbonylamino)benzoic acid (438 mg, 1.8 mmol), 3-CBz-aminopiperidine.HCl (500 mg, 1.8 mmol) and Et3N (0.89 ml, 5.5 mmol) in DMF (10 mL) was added HBTU (1.05 g, 2.8 mmol). The mixture was stirred 5 h at rt before being diluted with EtOAc (100 ml), washed with water (100 mL), brine (3×100 mL), dried (MgSO4), filtered and concentrated under reduced pressure. The mixture was purified by SiO2 chromatography (Hex / EtOAc 20 to 100% gradient) and afforded the title compound (765 mg, 1.69 mmol, 94%) as a colorless oil.
tert-butyl 4-(3-aminopiperidine-1-carbonyl)phenylcarbamate
[0172]
[0173]To a degassed solution of p-{[3-(Benzyloxycarbonylamino)-1-piperidyl]carbonyl}phenylamino 2,2-dimethylpropionate (765 mg, 1.69 mmol) in M...
example 2
Synthesis of (E)-N-(4-(3-((5-chloro-4-(1H-indol-3-yl)pyrimidin-2-ylamino)methyl)piperidine-1-carbonyl)phenyl)-4-(dimethylamino)but-2-enamide (Compound 101)
tert-butyl 3-((5-chloro-4-(1-(phenylsulfonyl)-1H-indol-3-yl)pyrimidin-2-ylamino)methyl)piperidine-1-carboxylate
[0182]
[0183]A solution of 3-(2,5-dichloropyrimidin-4-yl)-1-(phenylsulfonyl)-1H-indole (300 mg, 0.74 mmol), 1-Boc-3-(aminomethyl)piperidine (159 mg, 0.74 mmol) and diisopropylethylamine (0.13 mL, 0.74 mmol) in NMP (2.0 mL) was heated 25 min at 135° C. (mW). The mixture was diluted with EtOAc (30 mL), washed with water (3×5 mL), brine (5 mL), dried (MgSO4), filtered, and concentrated under reduced pressure. The residue was purified by SiO2 chromatography (DCM / EtOAc 0 to 15% gradient), and afforded the title compound (355 mg, 0.67 mmol, 85%) as a white solid.
5-chloro-4-(1-(phenylsulfonyl)-1H-indol-3-yl)-N-(piperidin-3-ylmethyl)pyrimidin-2-amine
[0184]
[0185]Trifluoroacetic acid (0.93 mL, 12.2 mmol) was added to a stirring solu...
example 3
Synthesis of (E)-3-((5-chloro-4-(1-(phenylsulfonyl)-1H-indol-3-yl)pyrimidin-2-yl)amino)-N-(4-(4-(dimethylamino)but-2-enamido)phenyl)piperidine-1-carboxamide (Compound 102)
5-chloro-4-(1-(phenylsulfonyl)-1H-indol-3-yl)-N-(piperidin-3-yl)pyrimidin-2-amine
[0194]
[0195]To a solution of 3-(2, 5-dichloropyrimidin-4-yl)-1-(phenylsulfonyl)-1H-indole (402 mg) in 1, 2-dimethoxylmethanol was added tert-butyl 3-aminopiperidine-1-carboxylate (200 mg, 1.0 equiv) and diisopropylethylamine (129 mg, 1.0 equiv). The solution was heated for 2 h at 120° C. The cooled solution was diluted with 100 mL of CHCl3 and i-PrOH(4:1) and then washed with water. After removing solvent, the crude product was dissolved in 10 mL CHCl3 and treated with 5 mL TFA. After stirring for 30 min at room temperature, the solvent was removed and the product was purified byby silica gel chromatography with CH2Cl2 / methanol (10:1) to give the product (350 mg, 76%).
3-((5-chloro-4-(1-(phenylsulfonyl)-1H-indol-3-yl)pyrimidin-2-yl)amin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| w/w | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com