Glycan-modified Anti-cd4 antibodies for HIV prevention and therapy
a technology of cd4 and glycan, which is applied in the field of glycan modification of antibodies and anticd4 antibodies, can solve the problems that no previous study provides the effect of glycan, and achieve the effects of improving activity, preventing infection of target cells, and inhibiting or/and treating infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
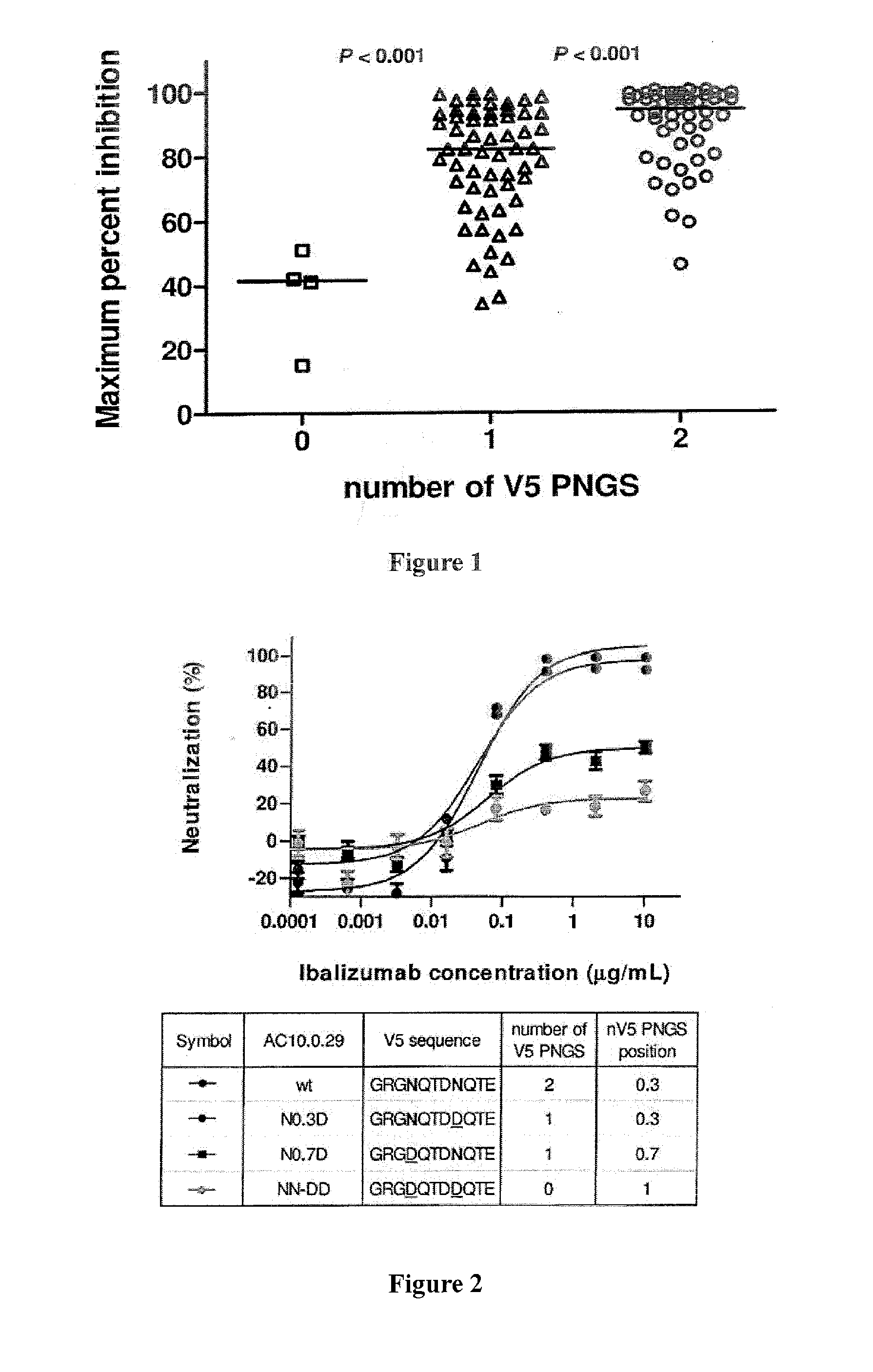
[0118]Sequence analysis of a panel of 118 viral isolates suggests that ibalizumab resistance was associated with the number of potential N-linked glycosylation sites (PNGS) in the V5 loop of gp120 (Pace et al., J. Acquir. Immune Defic. Syndr. Epub ahead of print: September 2012). As shown in FIG. 1, viruses having two N-linked glycosylation sites in V5 were sensitive to ibalizumab, whereas viruses having no N-linked glycosylation site in V5 were resistant. Bar indicates median. Interestingly, clinical viral isolates that have developed resistance to ibalizumab monotherapy also display a loss in a potential V5 glycosylation site (Toma et al., J. Virology 85(8): 3872-2880, 2011).
[0119]One wild-type virus (AC10.0.29) in the panel has two N-linked glycosylation sites in V5 and is naturally sensitive to ibalizumab. The V5 N-linked glycosylation sites in this virus were systematically deleted using site-directed mutagenesis, and the resulting mutant viruses became resistant to ibalizumab ...
example 2
Design of Ibalizumab Variants
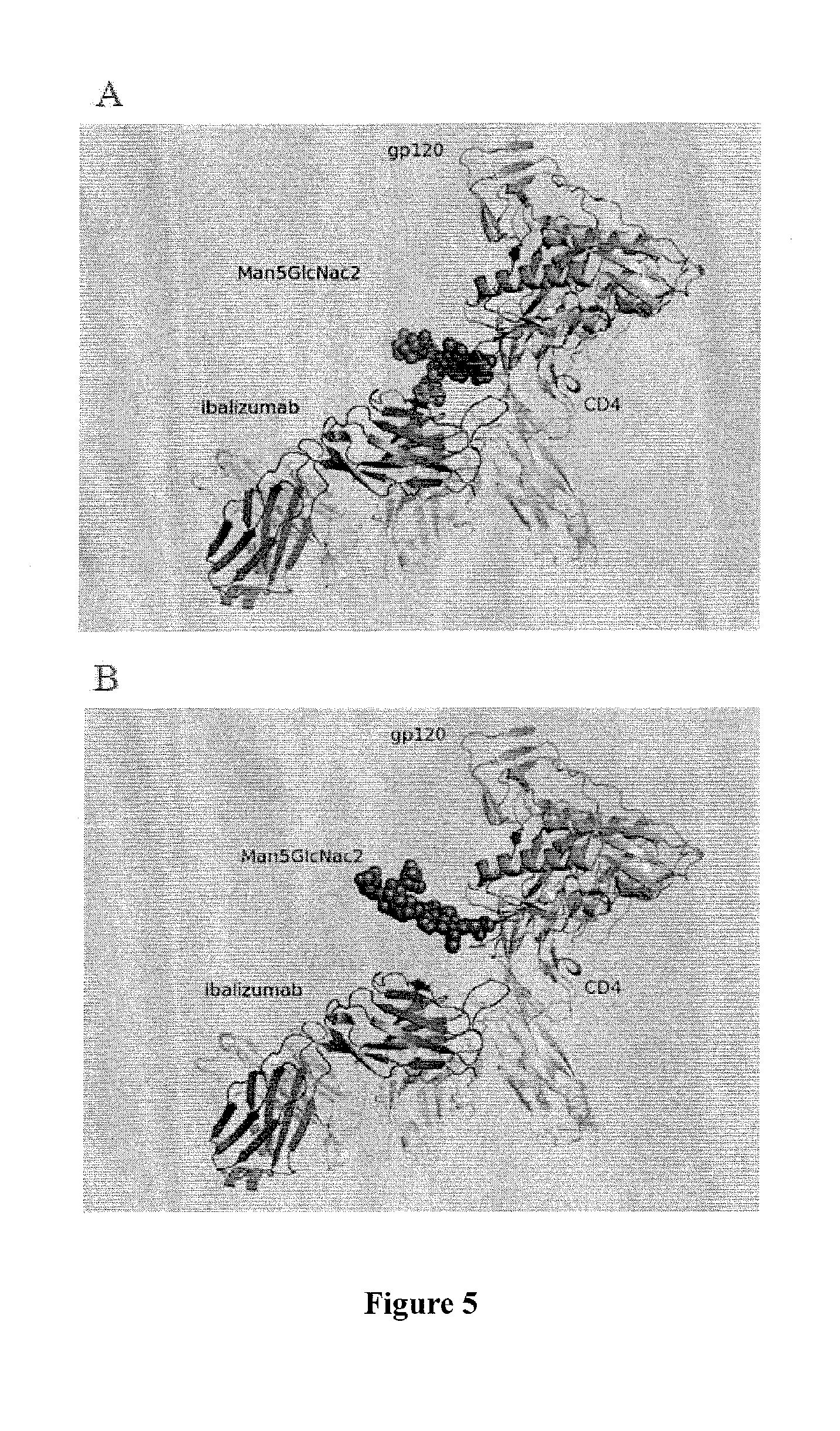
[0123]Studies of ibalizumab-resistant HIV-1 strains revealed that resistance is mainly conferred by the loss of glycan(s) from the V5 loop of HIV-1 Env gp120. To further explore the role of V5 glycosylation in ibalizumab susceptibility, we modeled the interactions between gp120, CD4 and ibalizumab using the structures reported to the Protein Data Bank (accession number 2NXY and 302D). The V5 N-terminal glycan is situated closest to the ibalizumab L chain (FIG. 5A), while the V5 C-terminal glycan is further away from ibalizumab (FIG. 5B). This model raises the possibility that the fit of the N-terminal glycan into the space between gp120 and ibalizumab exerts a mass effect on gp120, thereby disrupting its conformational changes (twists and turns) that are essential for HIV-1 entry into the target cell. This model also suggests that introduction of a similarly sized glycan into the ibalizumab L chain may boost its ability to inhibit entry of HIV-1 strains ...
example 3
CD4 Binding and HIV-1 Neutralization by LMs
[0125]We next evaluated the kinetics of binding of these ibalizumab LMs to human soluble CD4 (sCD4) by surface plasmon resonance. In a Biacore assay using sCD4 as the analyte, WT ibalizumab and LMs bound sCD4 with similar binding kinetics. The KD of six of these LMs bound to sCD4 was in the range of 0.19 nM to 0.8 nM (Table 1), and these numbers were within 2-fold of the KD of wild-type ibalizumab (0.43 nM). These data showed that the addition of an N-linked glycan at these select locations in the L chain of ibalizumab did not markedly affect its ability to bind CD4.
TABLE 1The binding kinetics of ibalizumab and its LMs to human CD4AntibodyKon (105 / Ms)Koff (10−5 / s)KD (0.1 nM)ibalizumab2.8124.3LM30E1.11514LM524.6163.5LM532.9134.5LM543.7277.3LM604.48.31.9LM653.34915LM672.5208LM765.0142.8
[0126]We next explored the HIV-1 neutralizing capacity of the LMs compared to WT ibalizumab. To this end, we tested the LMs against a panel of HIV-1 viruses th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com