Materials and methods for treating disorders associated with sulfatase enzymes
a technology of sulfatase enzyme and materials, which is applied in the field of materials and methods for treating disorders associated with sulfatase enzyme, can solve the problems of dementia and death, limited treatment options, and decline in learning ability, and achieve the effect of speeding up and flexible scaling up manufacturing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Produce SGSH and SGSH-Lectin Fusion Proteins
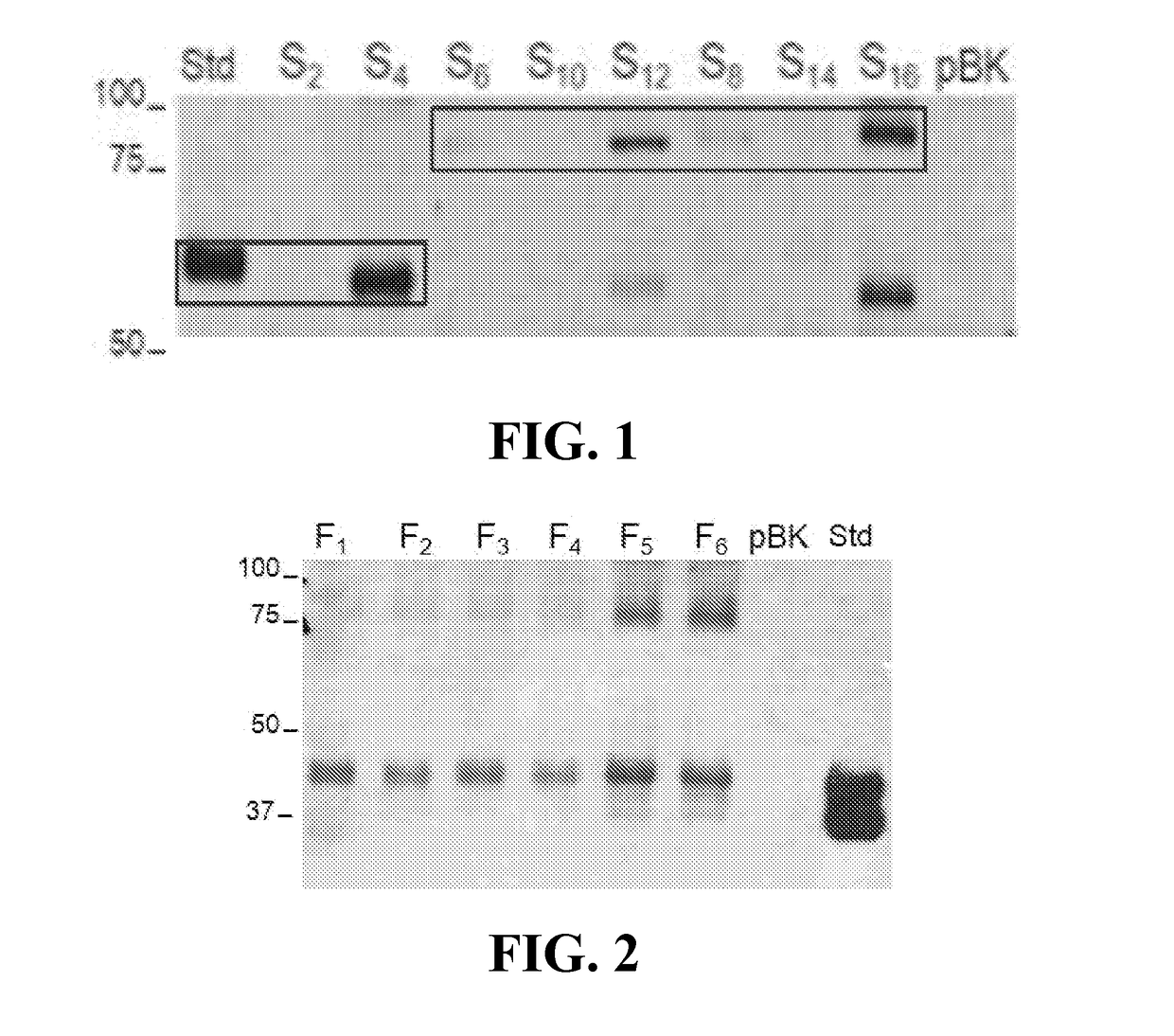
[0102]Construct design and plant-based expression. Sixteen gene constructs encoding SGSH and SGSH fusions with RTB and NBB (Table 6) were developed and expressed transiently in N. benthamiana leaves. Variants assessing signal peptides (human SGSH vs. plant-derived signal peptide), codon usage (SGSH sequence vs tobacco codon optimized), and fusion orientation were compared for product yield and quality (FIG. 1). Constructs were introduced into Agrobacterium tumefaciens, and induced cultures were vacuum infiltrated into leaves of intact plants and incubated for 2 to 5 days prior to harvest (Medrano et al., 2009). All constructs produced recombinant products of the expected sizes (56 kDa for SGSH; ˜91 kDa for lectin-SGSH fusions) that cross-reacted with anti-RTB, anti-His-tag, and anti-SGSH antibodies as appropriate (e.g., see FIG. 1). All constructs that used the native human signal peptide showed significantly lower product than those using...
example 2
Assess SGSH Enzyme and Carbohydrate-Binding Activity of Plant-Made SGSH and SGSH-Lectin Fusions
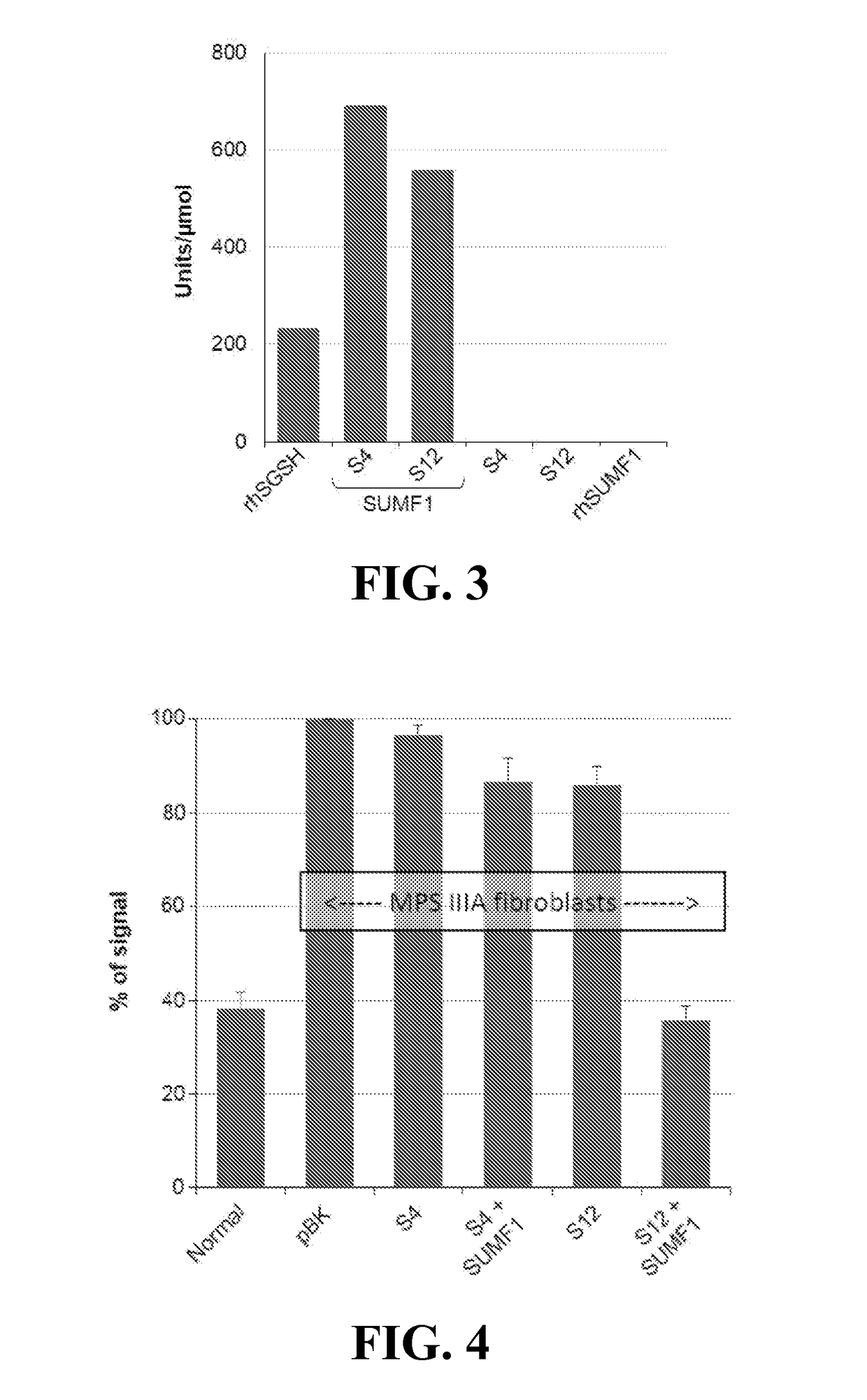
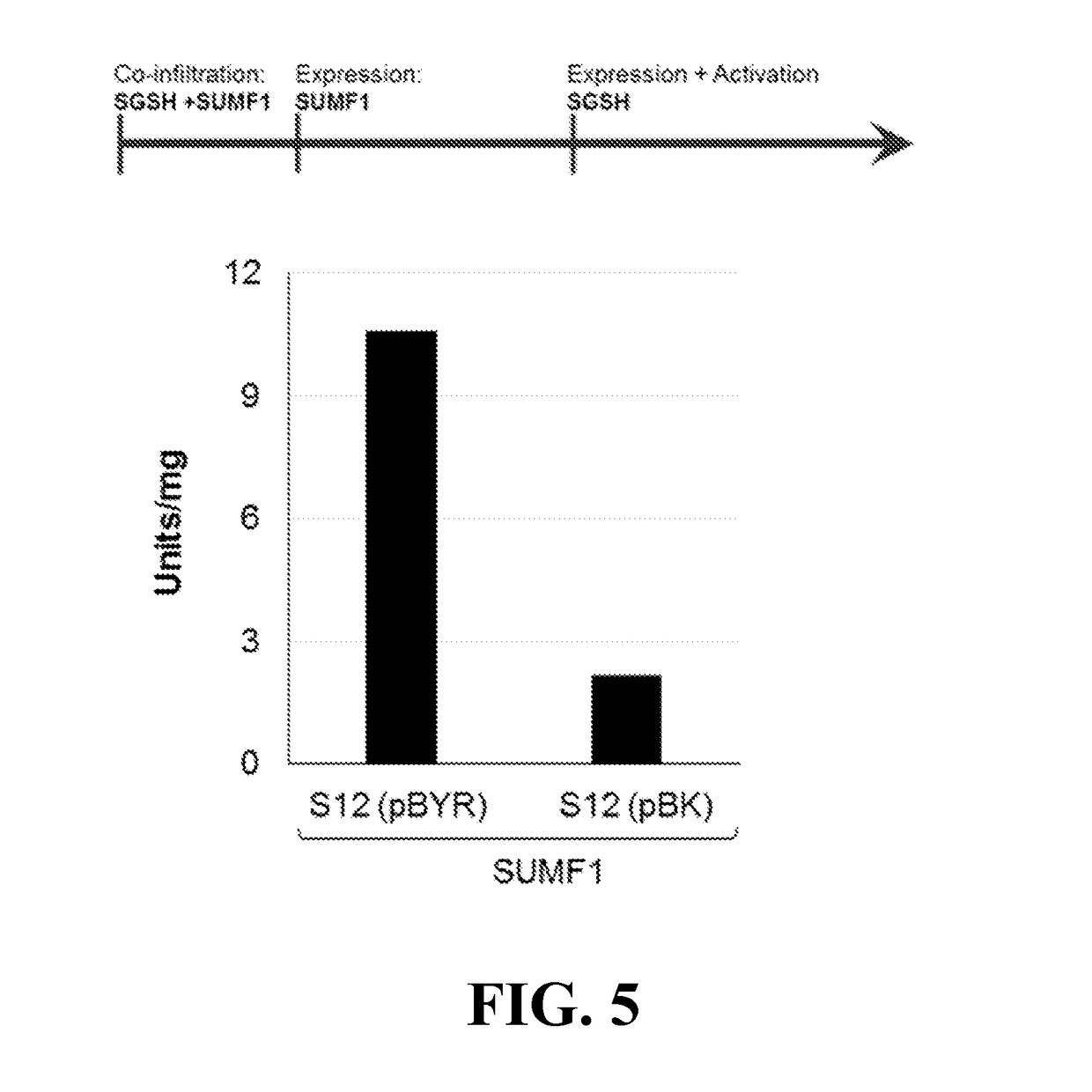
[0103]Assessment of SGSH activity. Plant tissues expressing S4, S12 and S16 constructs were used for extraction and initial purification of the SGSH and SGSH-fusion proteins. Several extraction buffers and clarification strategies were tested with the goal to obtain initial test material to assess activity. Leaf extracts were subjected to an initial affinity chromatography enrichment step (Nickel IMAC was used for the His-tagged S4; lactose resin for the S12 RTB fusion, and N-acetyl-galactosamine resin for the S16 NBB fusion). Recovery of the S12 and S16 products on selective sugar affinity columns confirmed lectin activity of the products. These proteins were quantified and used to assess SGSH activity based on the standard 2-step fluorometric assay as described (Karpova et. al., 1996) and using recombinant human SGSH (Novoprotein; made in HEK293 cells) as control proteins. No sulfamidase...
example 3
Demonstrate Uptake, Lysosomal Delivery, and Reduction of “Disease Substrate” in MPS IIIA Cultured Cells Treated with SGSH and SGSH-Lectin Fusions
[0106]MPS IIIA patients are deficient in SGSH activity leading to pathological accumulation of sulfated glucosaminoglycans (GAGs) with cellular phenotypes including elevated GAGs and increased lysosomal volume per cell. As a further demonstration that the plant-produced SGSH was fully functional following modification by SUMF1, MPS IIIA patient fibroblasts (GM01881) were treated with plant-produced SGSH (S4) or SGSH-RTB (S12) that were expressed in the presence and absence of co-expressed SUMF1 (FIG. 4). S12 (SGSH:RTB) produced in the presence of SUMF1 effectively reduced GAG content and lysosomal volume to “normal” levels. SGSH alone (S4+ / −SUMF1) was not corrective indicating that lectin-based delivery as well as FGly activation are critical in phenotype correction. These results indicate that RTB effectively delivers active SGSH to the si...
PUM
| Property | Measurement | Unit |
|---|---|---|
| speed | aaaaa | aaaaa |
| flexible | aaaaa | aaaaa |
| lysosomal volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com