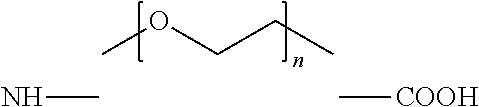
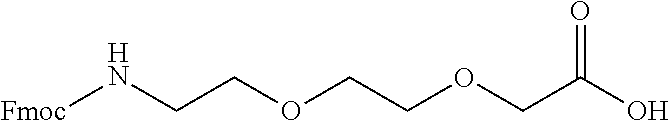
Peptide analogs
a technology of analogs and peptides, applied in the field of peptide analogs, can solve the problems of inability to perform antagonists as well in in vivo models, inability to achieve the effects of inducible side effects, and inability to detect abnormal liver tests in some patients, so as to prevent unwanted side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0329]Based on evidence that CLR / RAMP receptors modulate a number of central nervous system (CNS) and peripheral vascular activities, many analogs of adrenomedullin, CGRPs and intermedin have been synthesized and studied. Thus far, known synthetic analogs of these peptide hormones exhibit receptor-activation activities either comparative or inferior to wild type ligands. Likewise, synthetic antagonistic analogs of these peptide hormones (e.g., CGRP8-37 and ADM22-52; Rovero, P. et al. (1992) Peptides 13:1025-1027) exhibit mild bioactivities and are mainly specific for one of the three CLR / RAMP receptors (i.e., CLR / RAMP1, 2, and 3 receptors). No pan-specific superagonist or antagonist as well as CLR / RAMP1- or 2-selective superagonist and super-antagonist was previously known.
[0330]In humans and other mammals, including rodents, there are three CLR / RAMP receptors for adrenomedullin, CGRPs, and intermedin. These are CLR / RAMP1, 2, and 3 receptors, which are expressed throughout the vascu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com