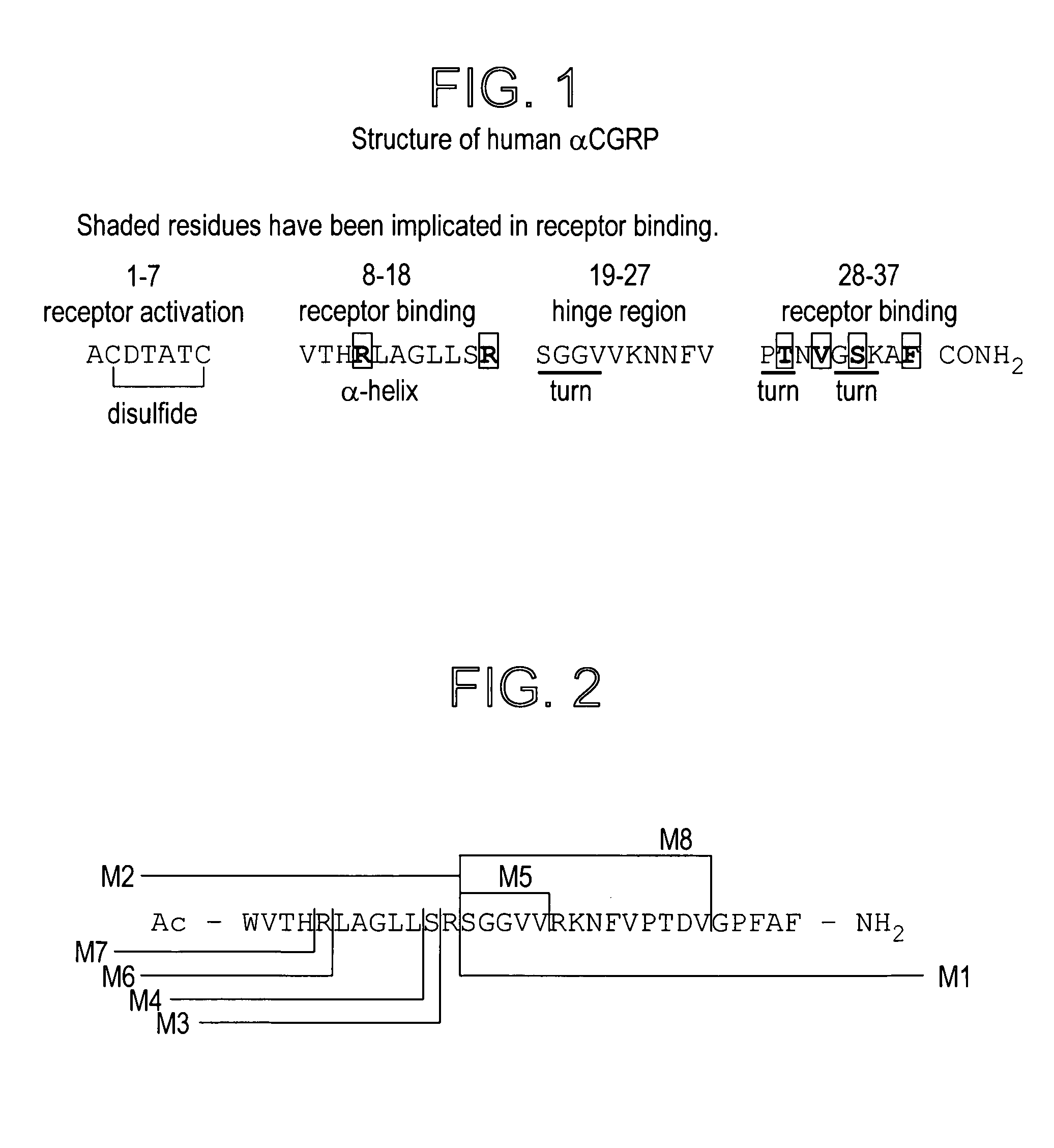
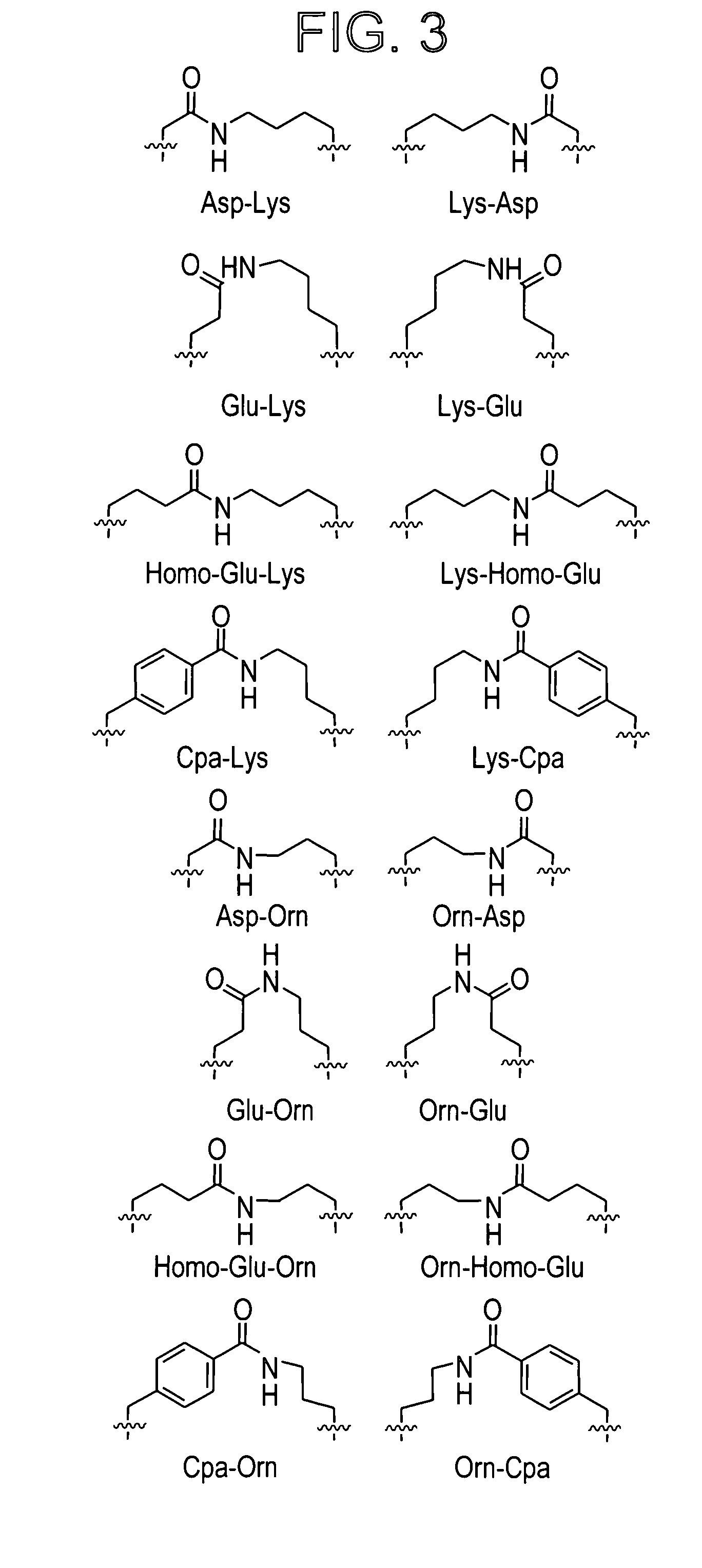
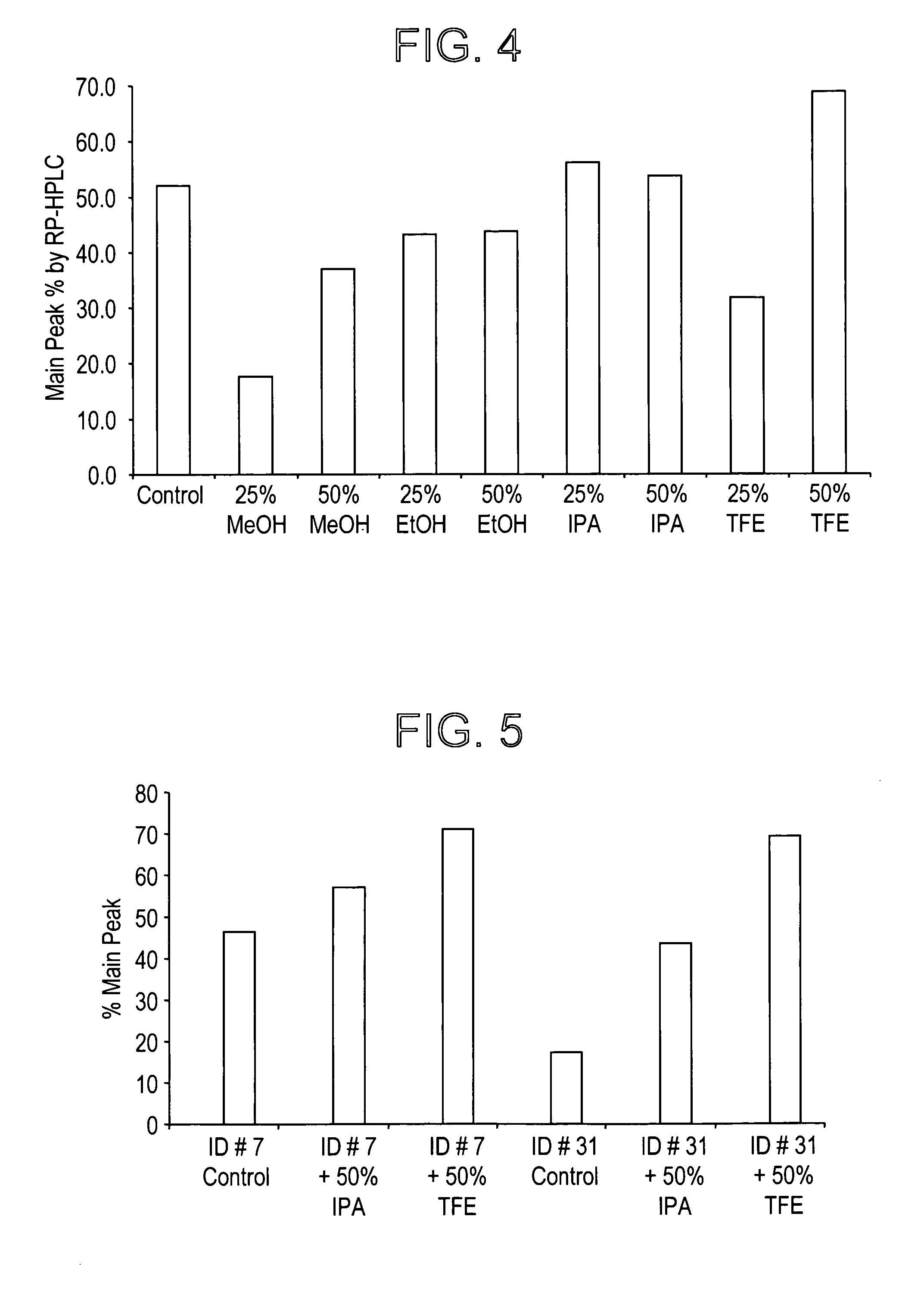
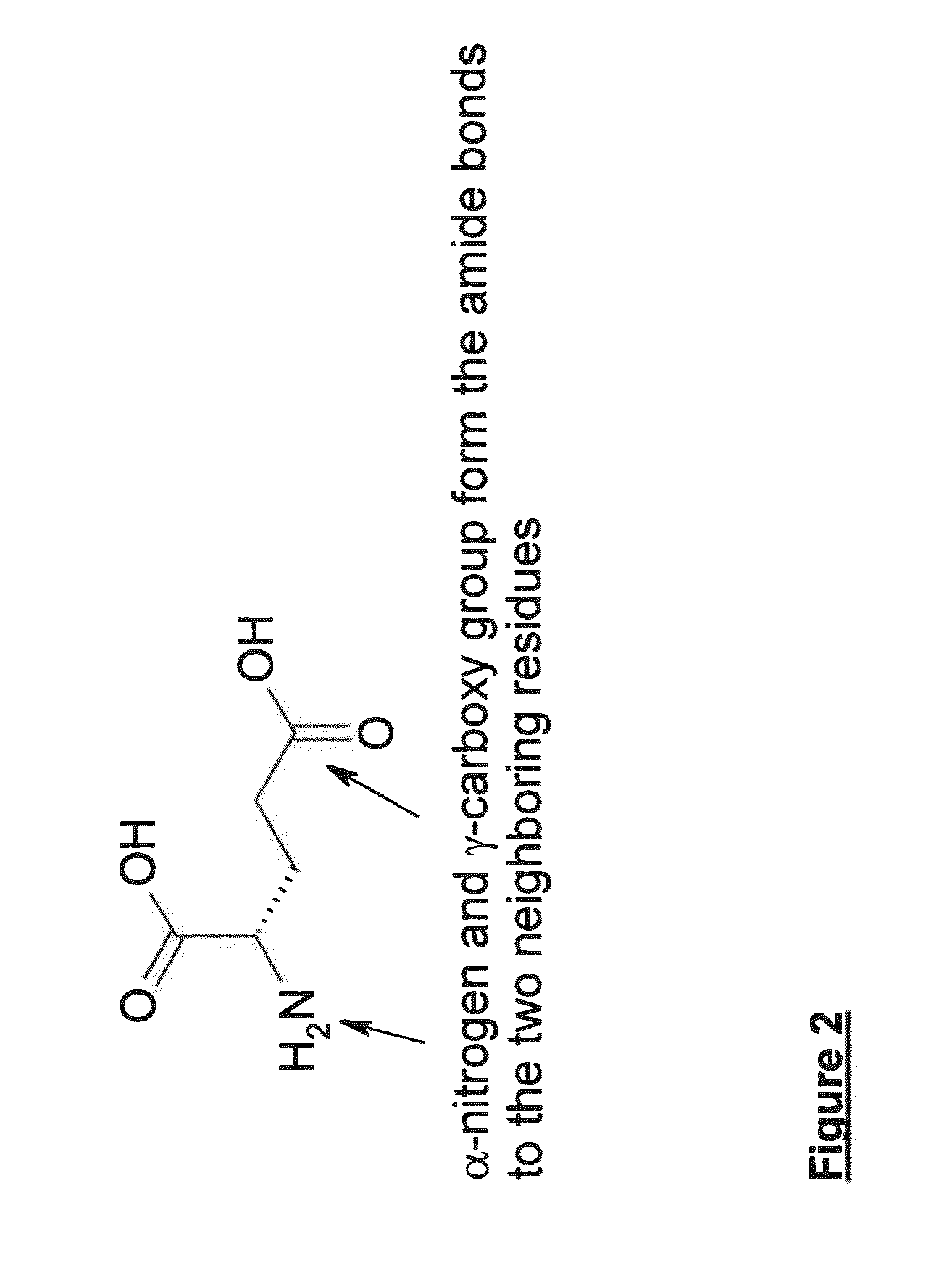
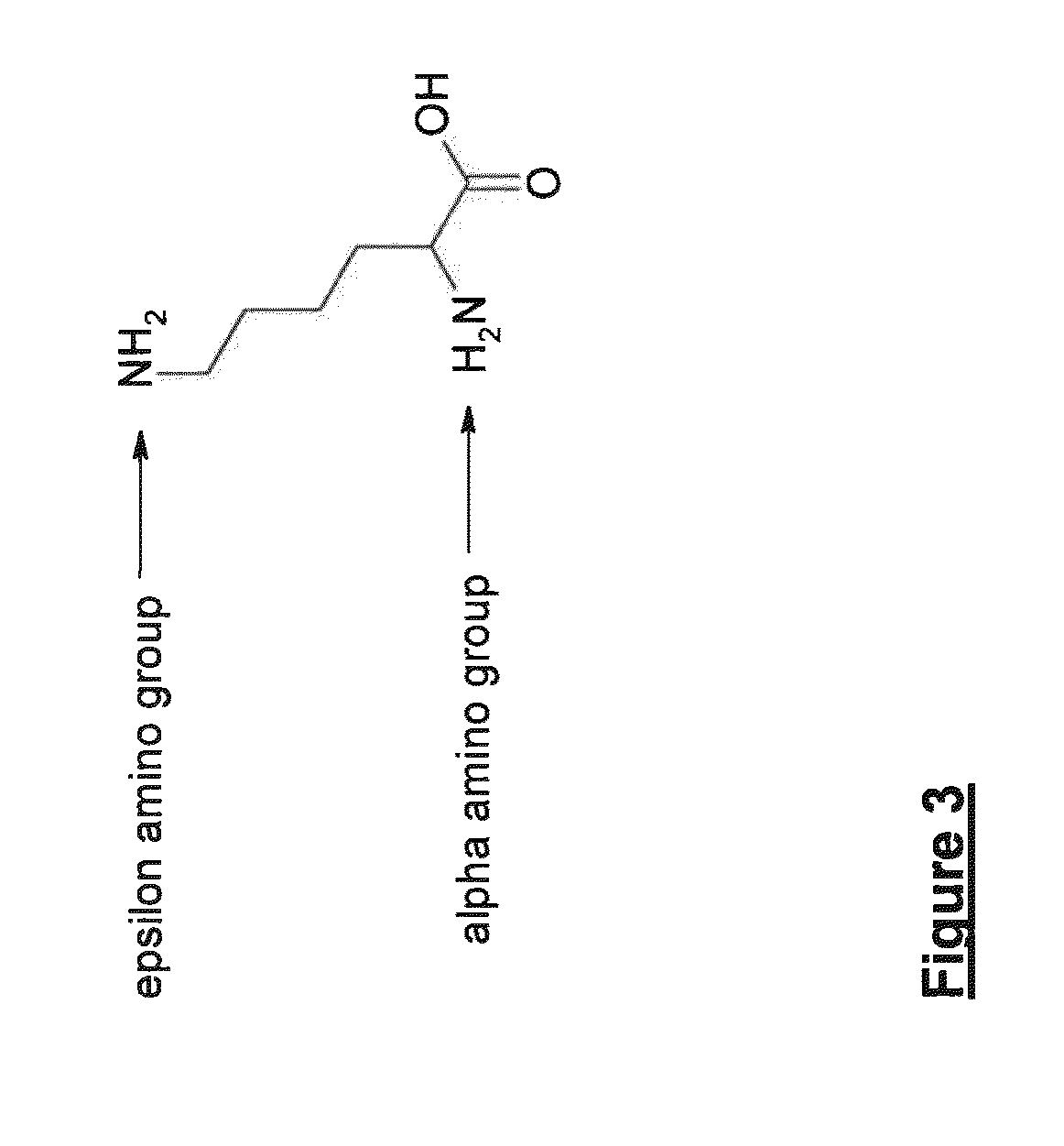
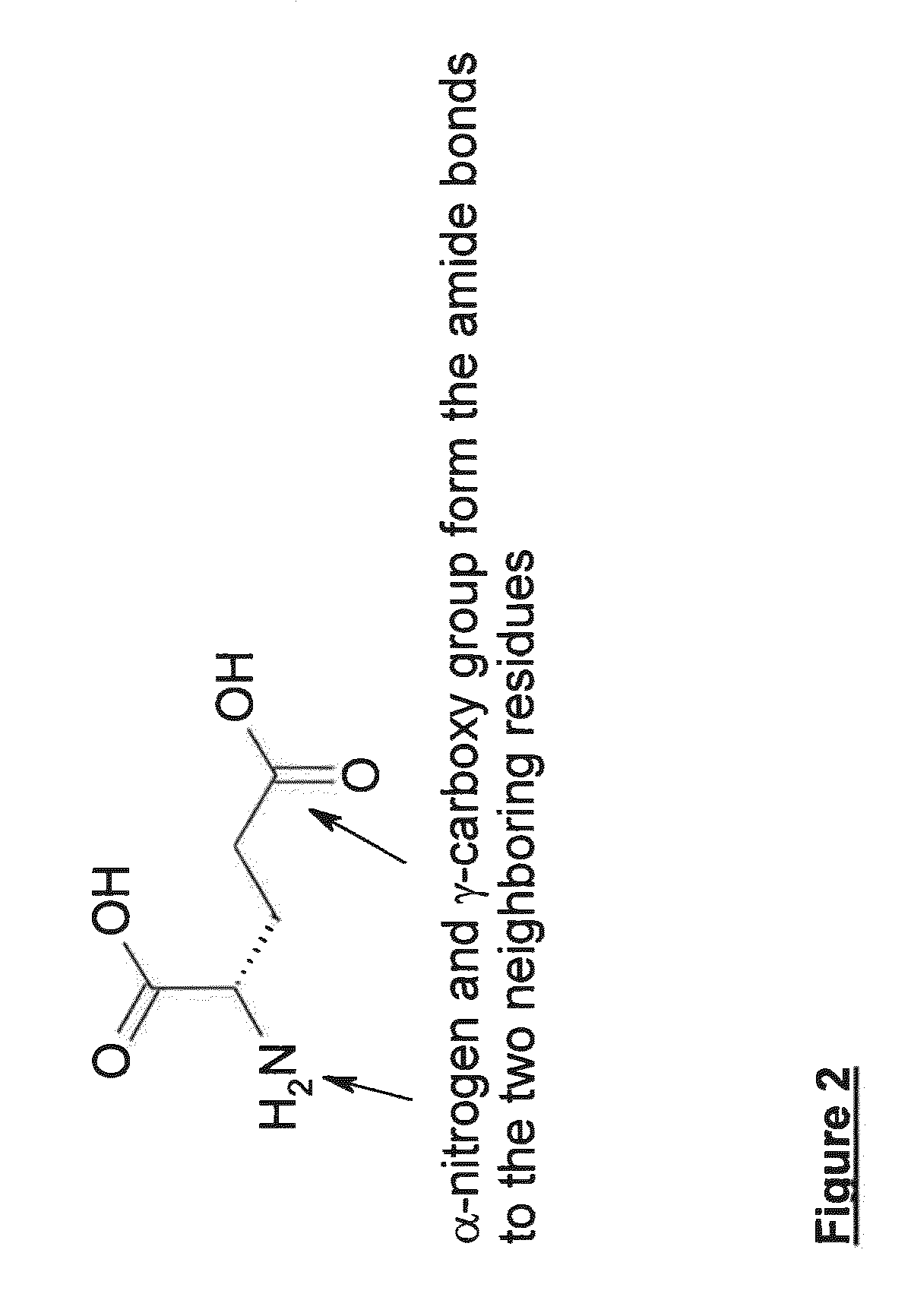
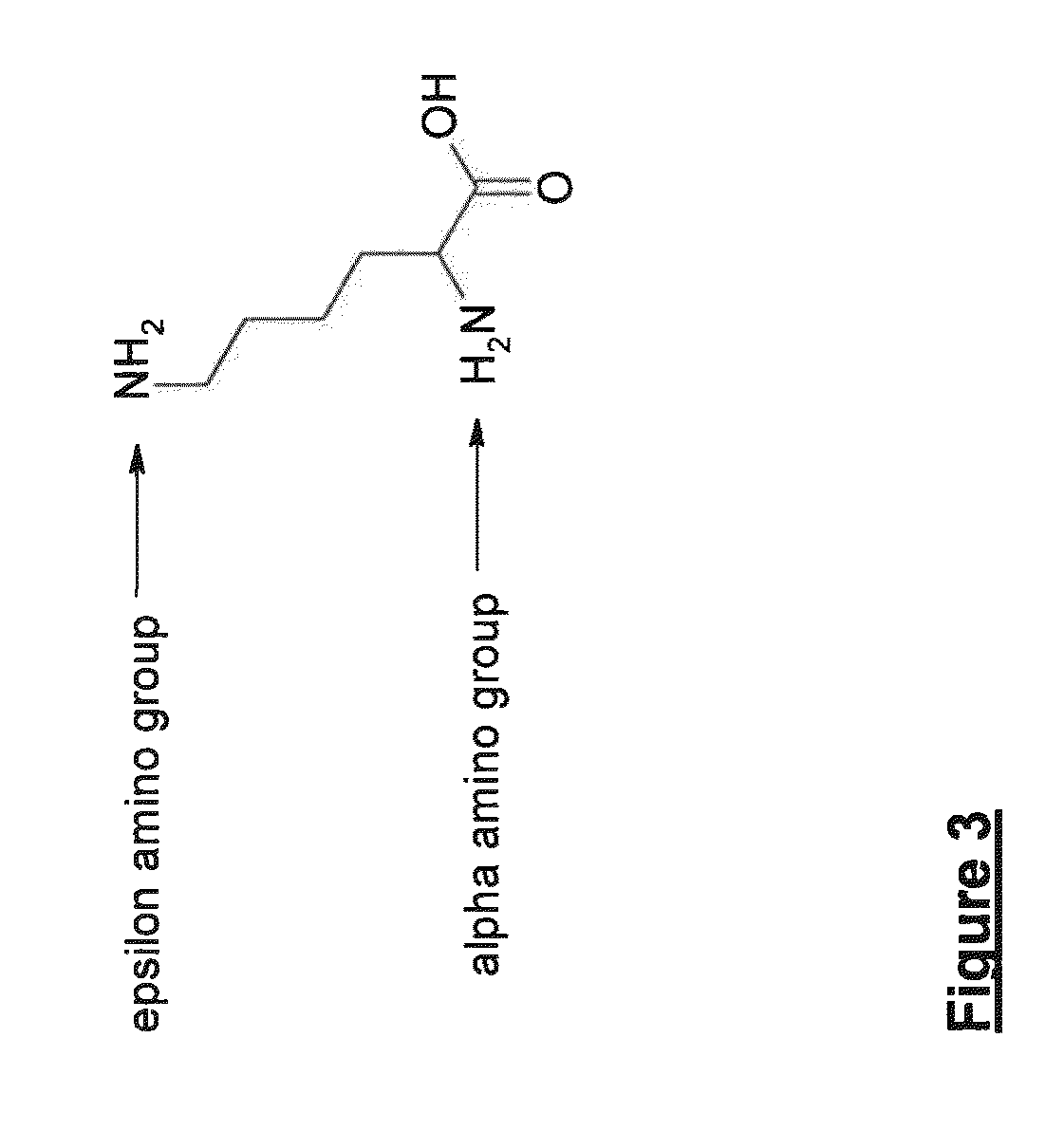
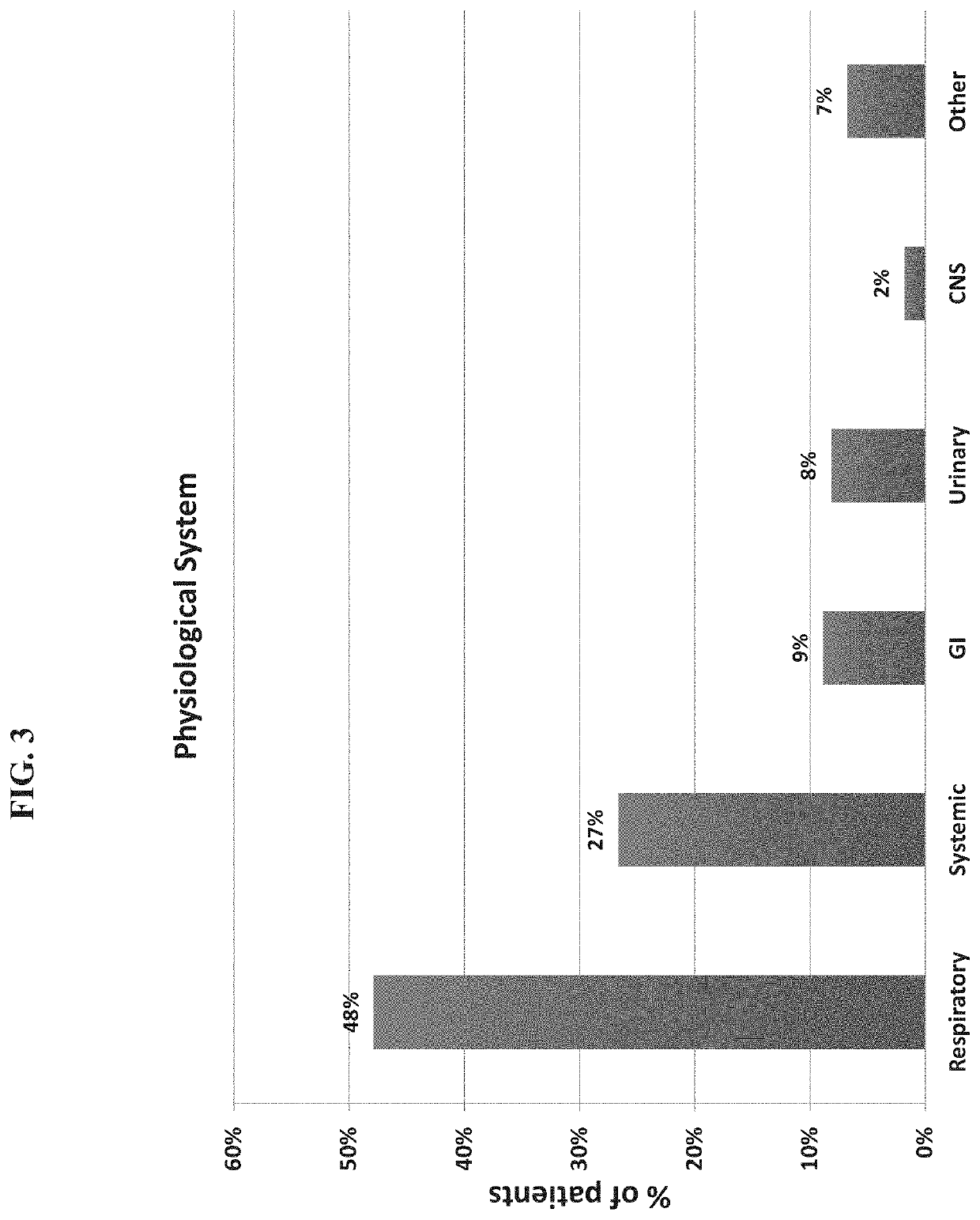
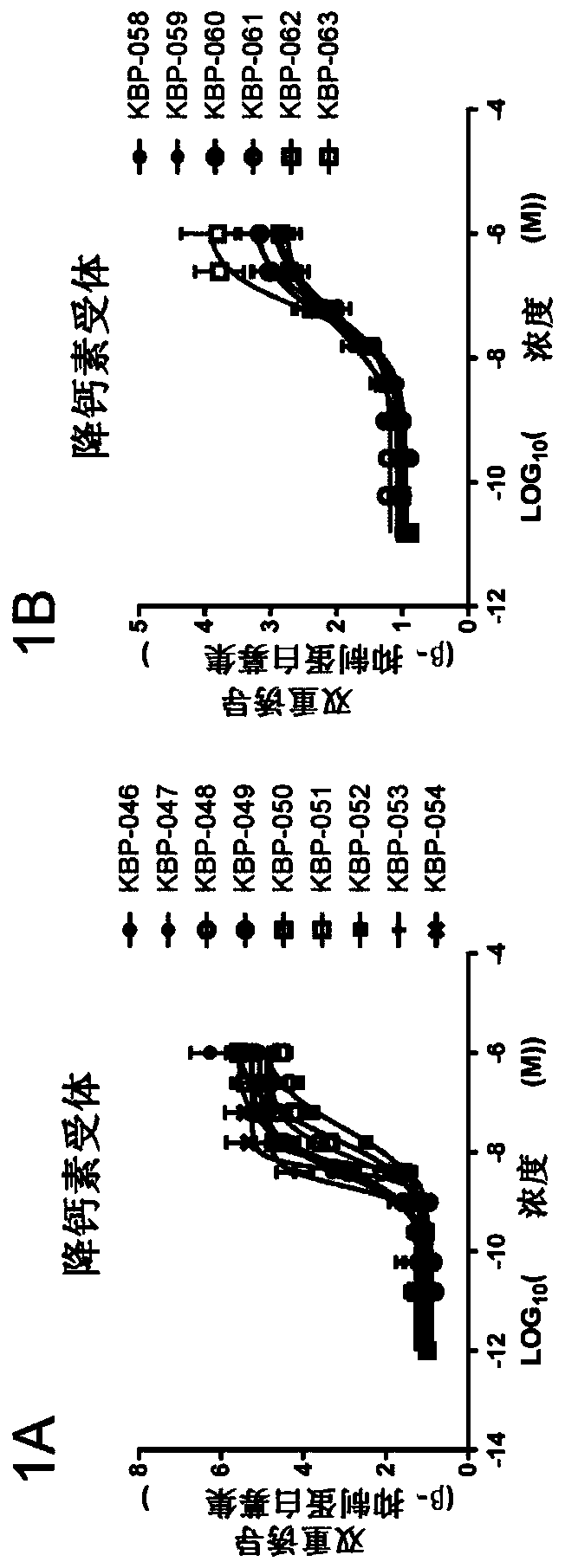
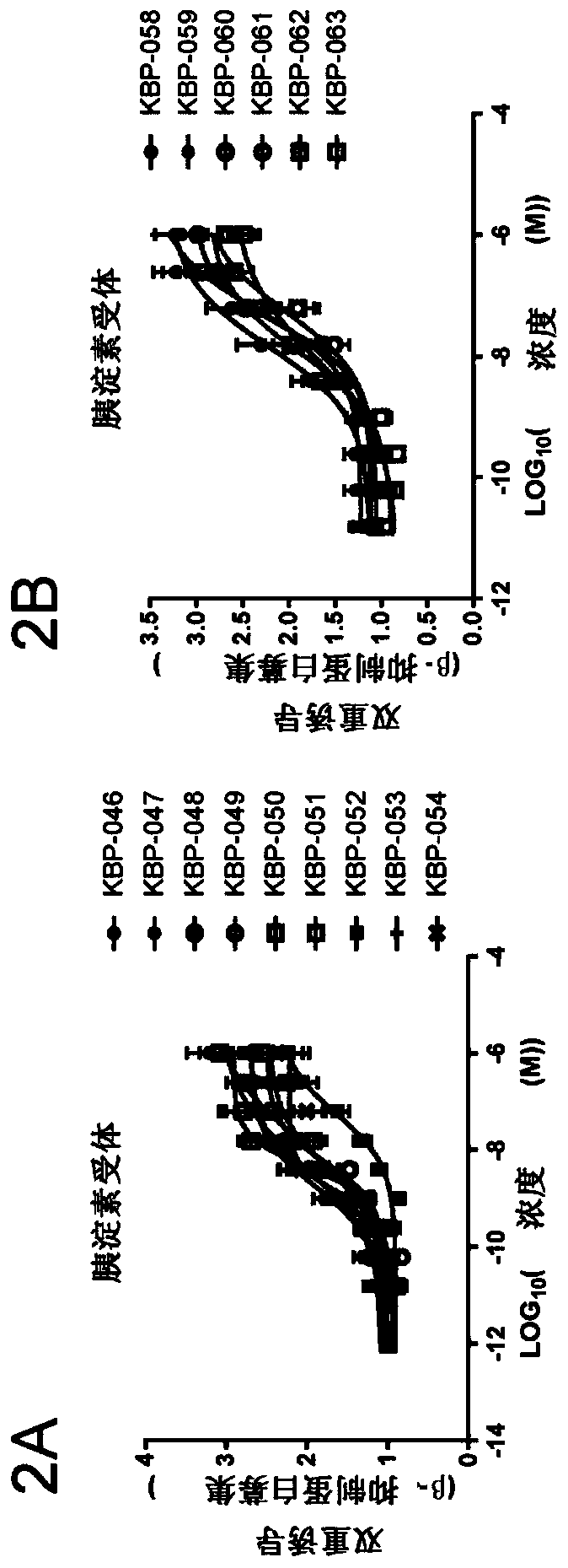
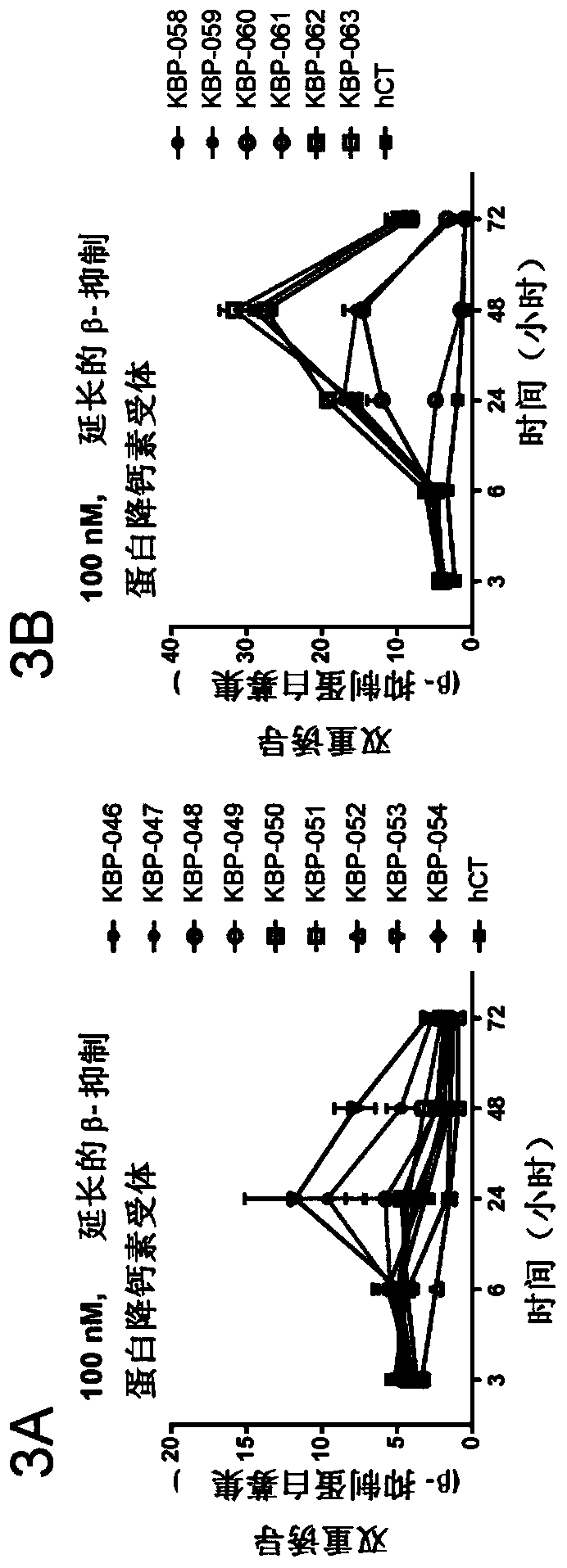
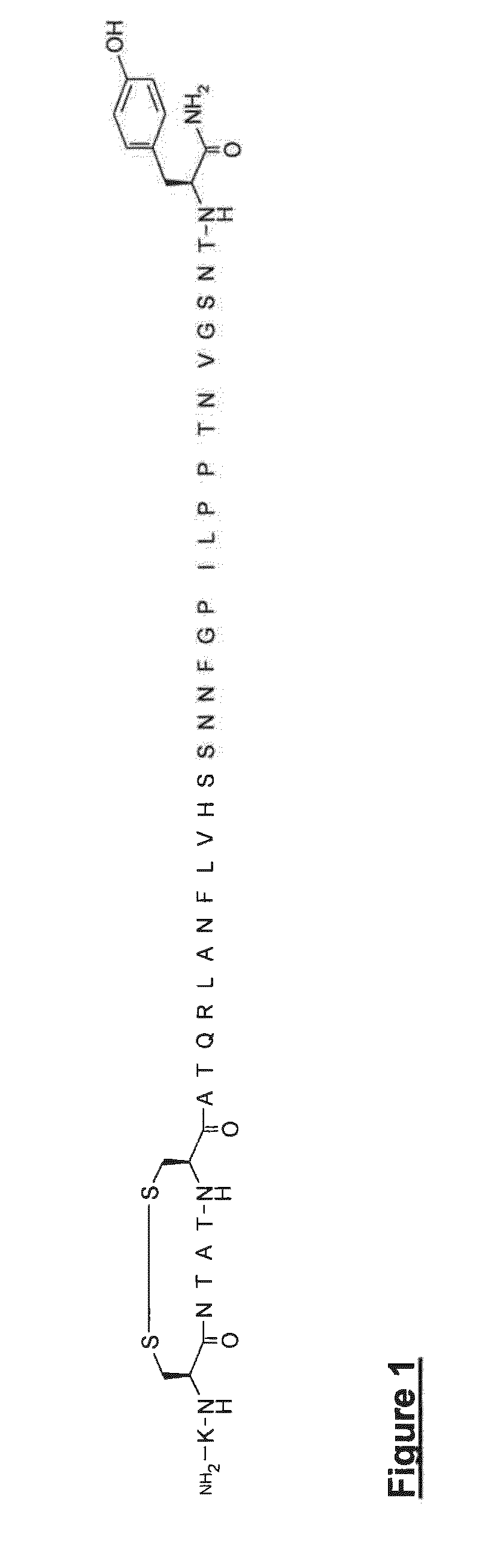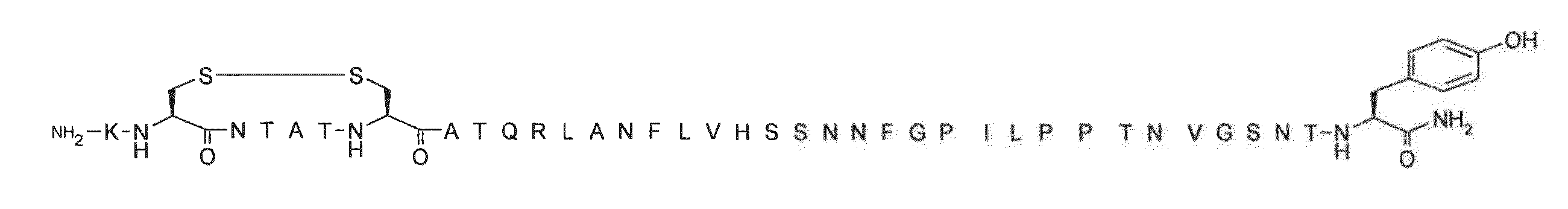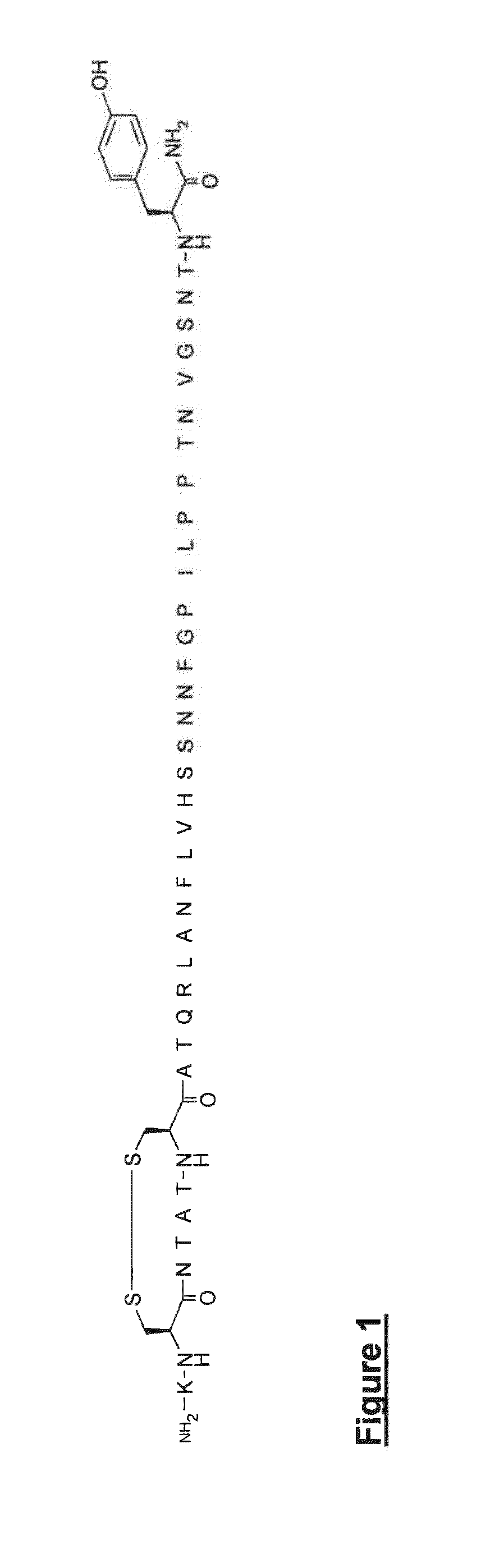Patents
Literature
32results about "Calcitonin gene related peptide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
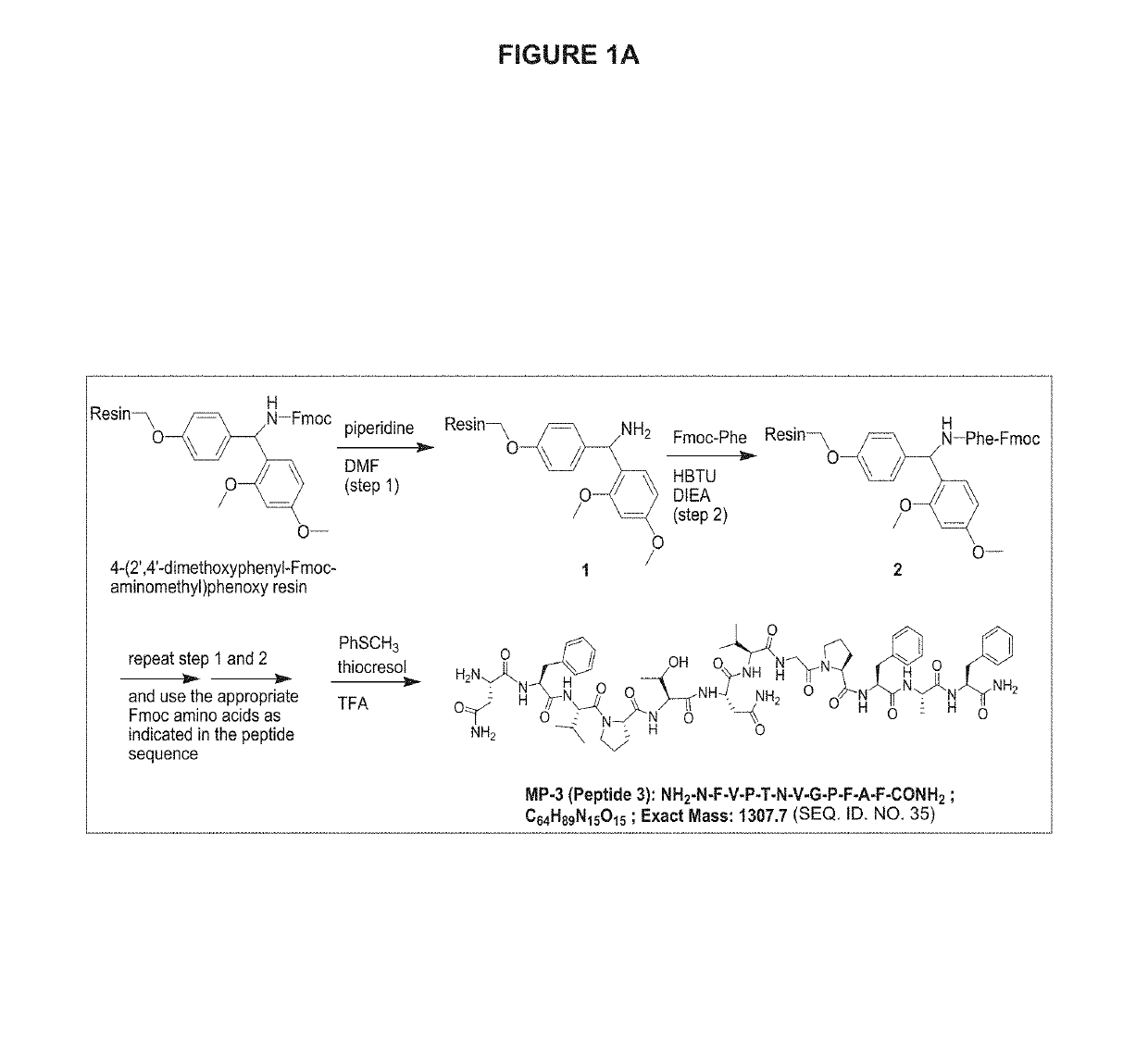
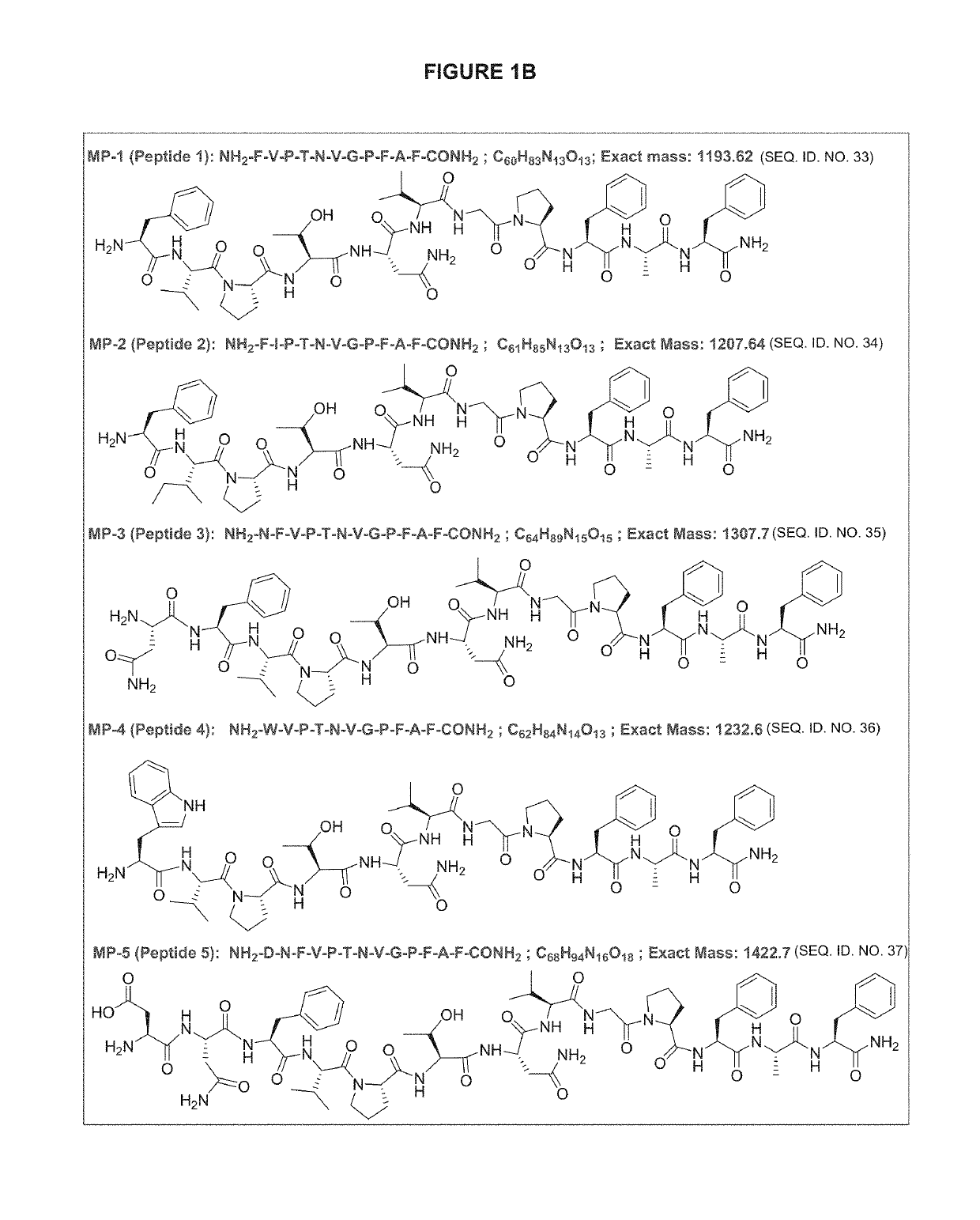
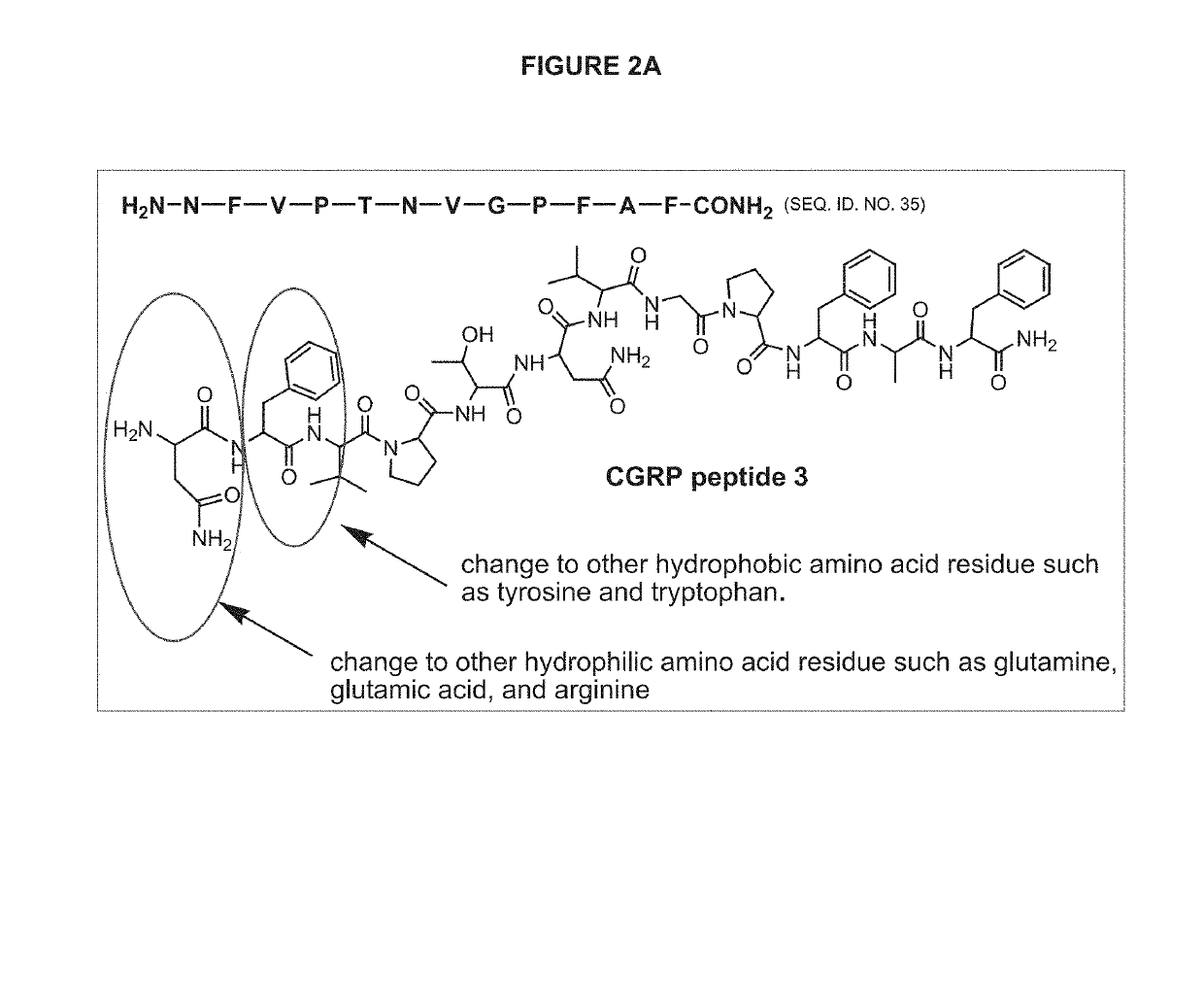
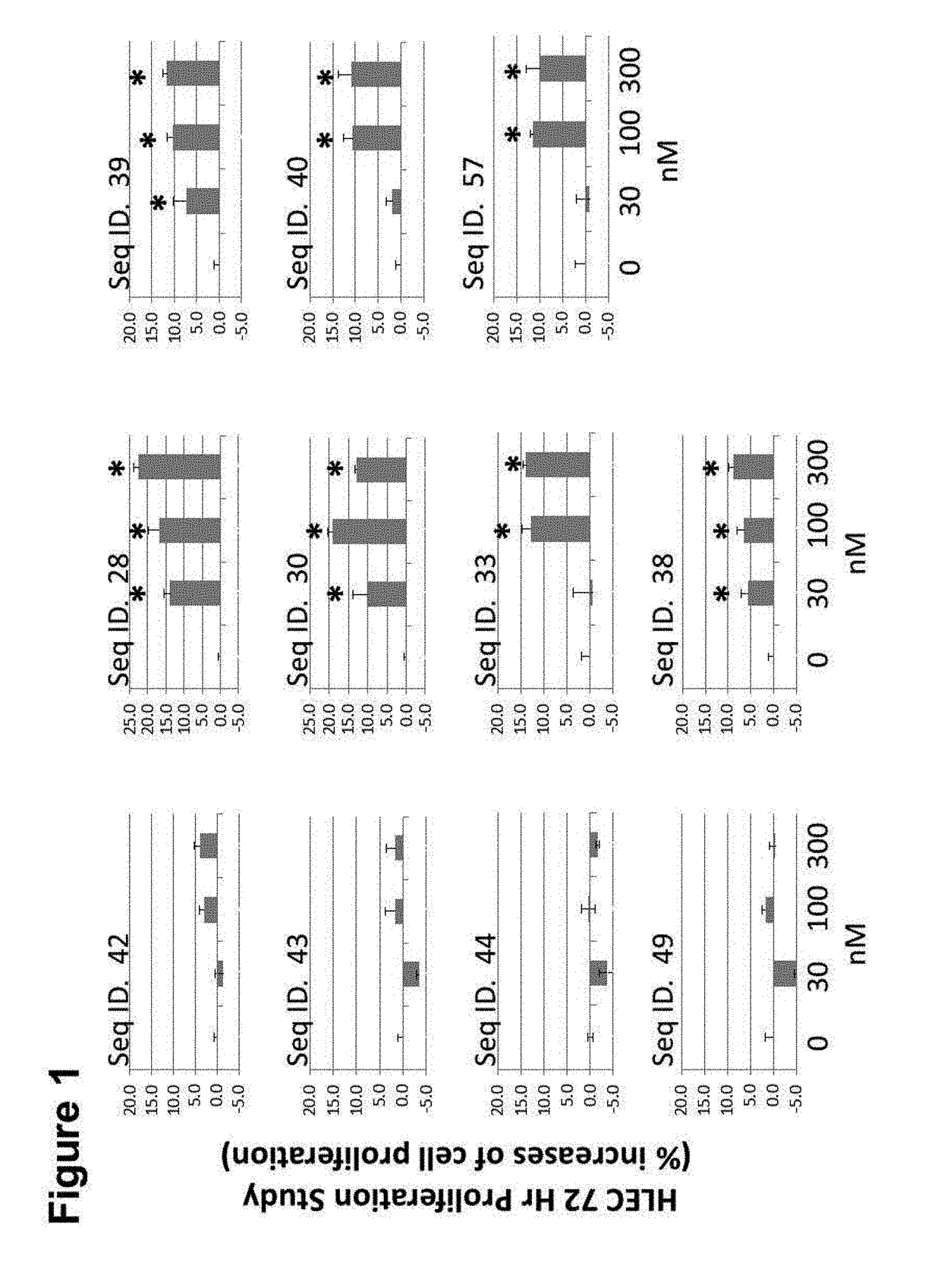
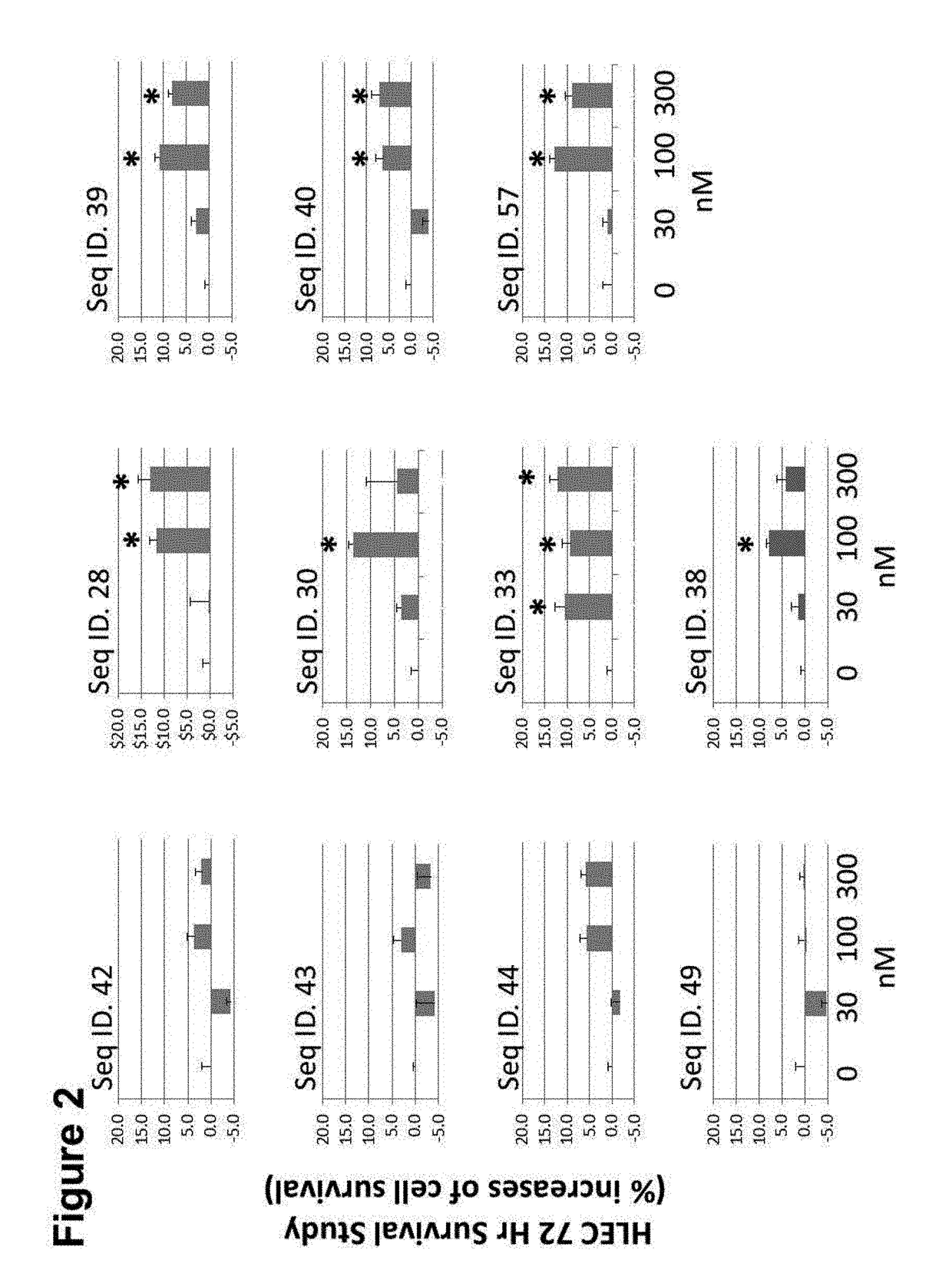
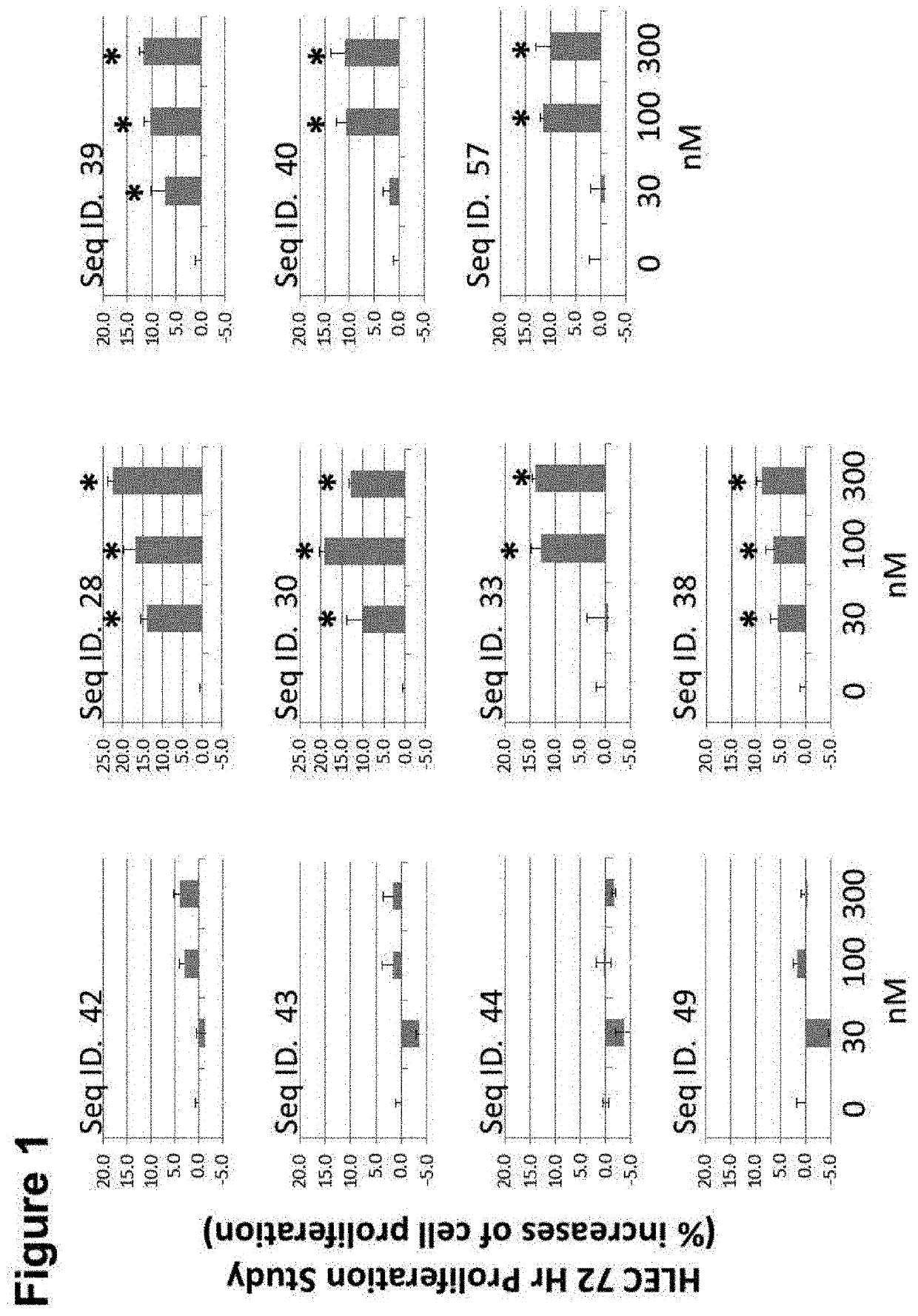
CGRP peptide antagonists and conjugates
InactiveUS8168592B2Extended half-lifeLow immunogenicityNervous disorderPeptide/protein ingredientsMigraineAntagonist
Disclosed is a composition of matter that involves a CGRP peptide antagonist. A pharmaceutical composition is disclosed that comprises the composition of matter and a pharmaceutically acceptable carrier, which can be configured for administration to a patient. Also disclosed is a method of producing the composition of matter. Methods of treating, preventing or mitigating migraine, are also disclosed.
Owner:AMGEN INC
CGRP peptide antagonists and conjugates
InactiveUS20080020978A1Preventing and mitigating migraineExtended half-lifeAntibacterial agentsNervous disorderMigraineAntagonist
Disclosed is a composition of matter that involves a CGRP peptide antagonist. A pharmaceutical composition is disclosed that comprises the composition of matter and a pharmaceutically acceptable carrier, which can be configured for administration to a patient. Also disclosed is a method of producing the composition of matter. Methods of treating, preventing or mitigating migraine, are also disclosed.
Owner:AMGEN INC
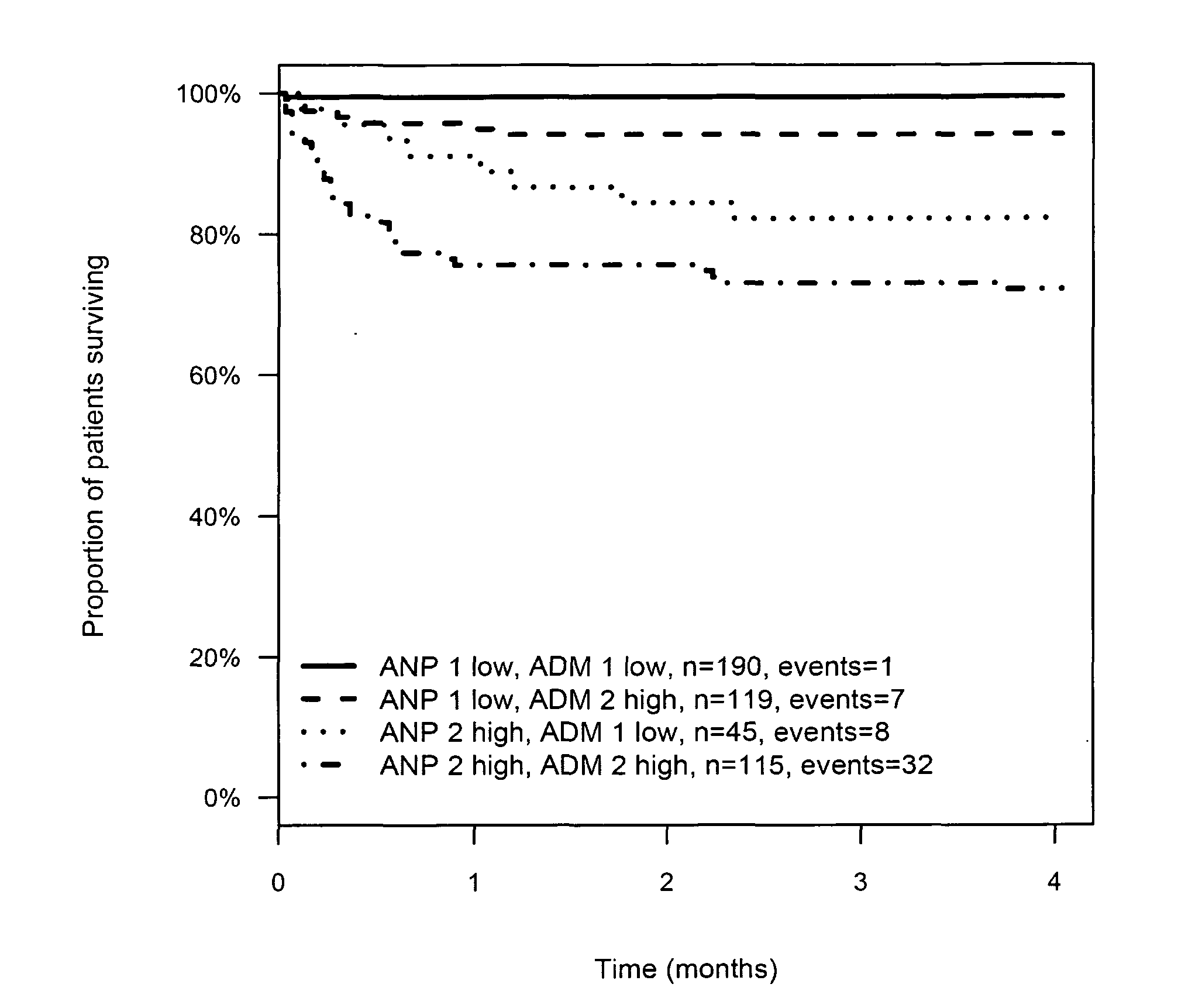
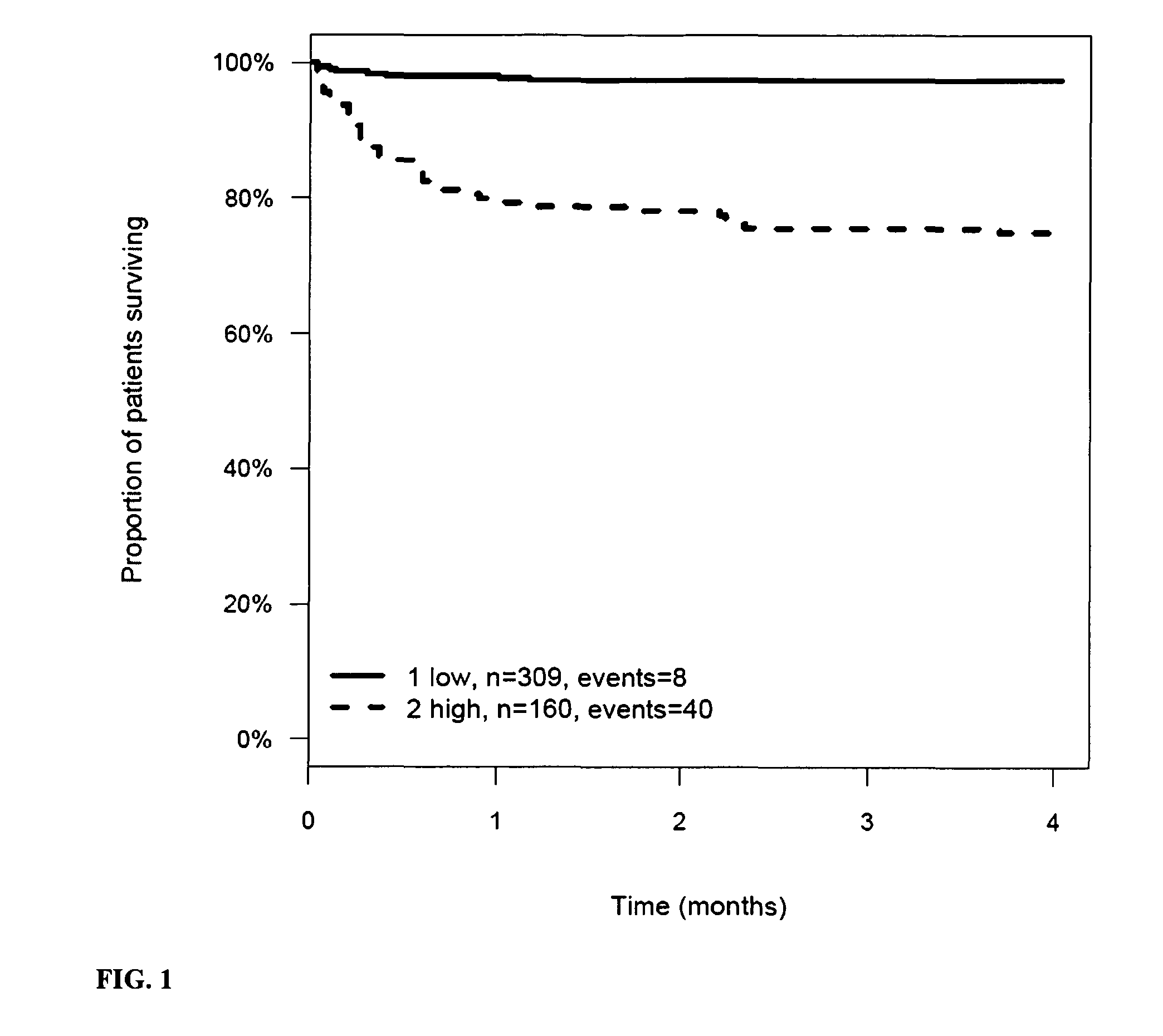
Prognosis and risk assessment in stroke patients by determining the level of marker peptides
InactiveUS20110263821A1Increase probabilityChemiluminescene/bioluminescenceLibrary screeningCalcitoninCancer risk assessment
The present invention relates to a method for prognosis of an outcome or assessing the risk of a patient having suffered a stroke or a transient ischemic attack, comprising the determination of the level of at least one marker peptide in said sample said marker peptide selected from the group comprising ANP, AVP, ADM, ET-1, troponin, CRP, calcitonin and hGH or fragments thereof or its precursor or fragments thereof and attributing the level of said at least one marker peptides its precursor or fragments thereof with the prognosis of an outcome or assessing the risk for said patient.
Owner:BRAHMS GMBH
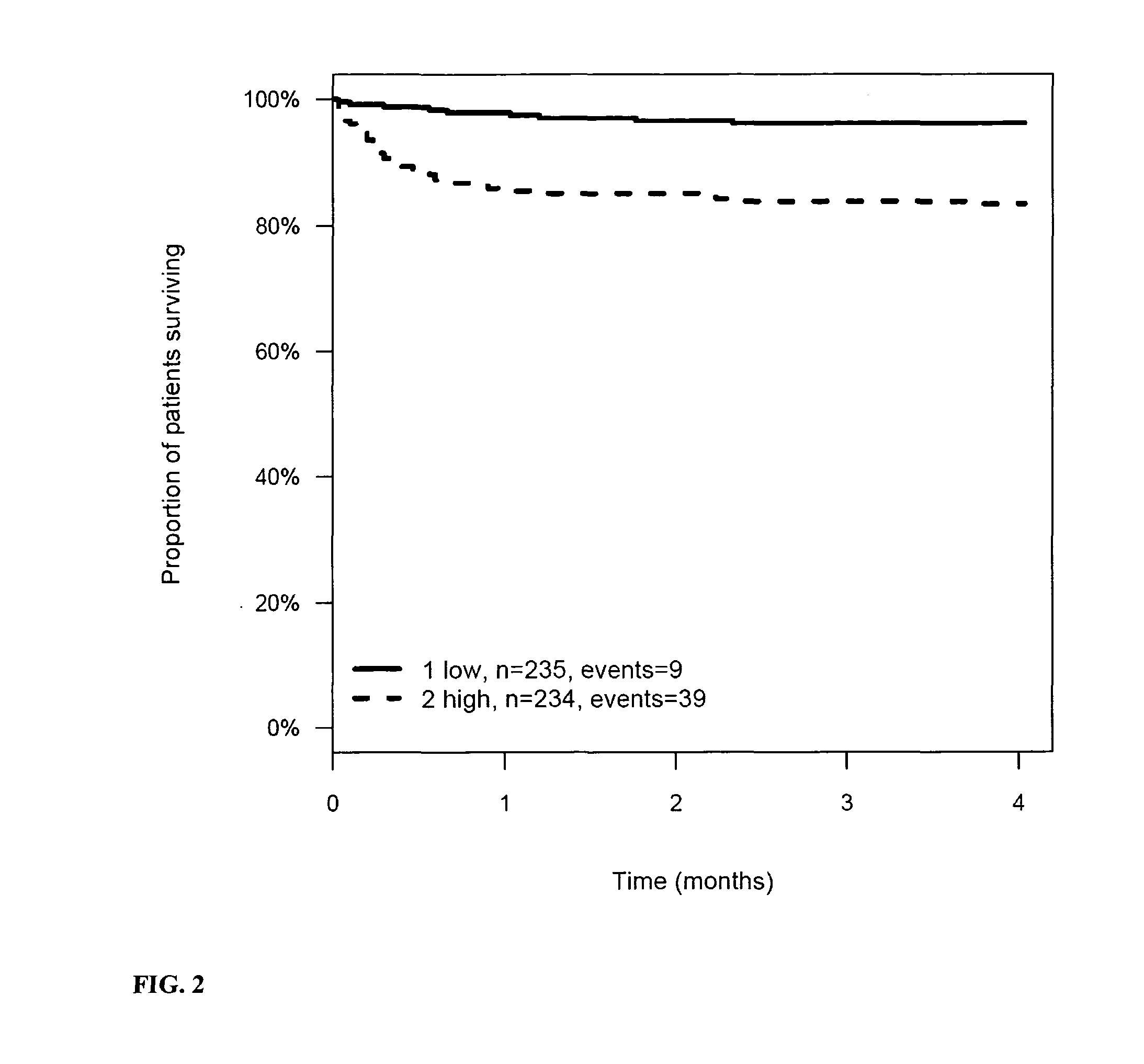
Protein signatures for distinguishing between bacterial and viral infections
ActiveUS20190120837A1Increased mortalityHigh diagnostic sensitivityTumor necrosis factorImmunoglobulins against cytokines/lymphokines/interferonsBacteroidesProtein Feature
Owner:MEMED DIAGNOSTICS
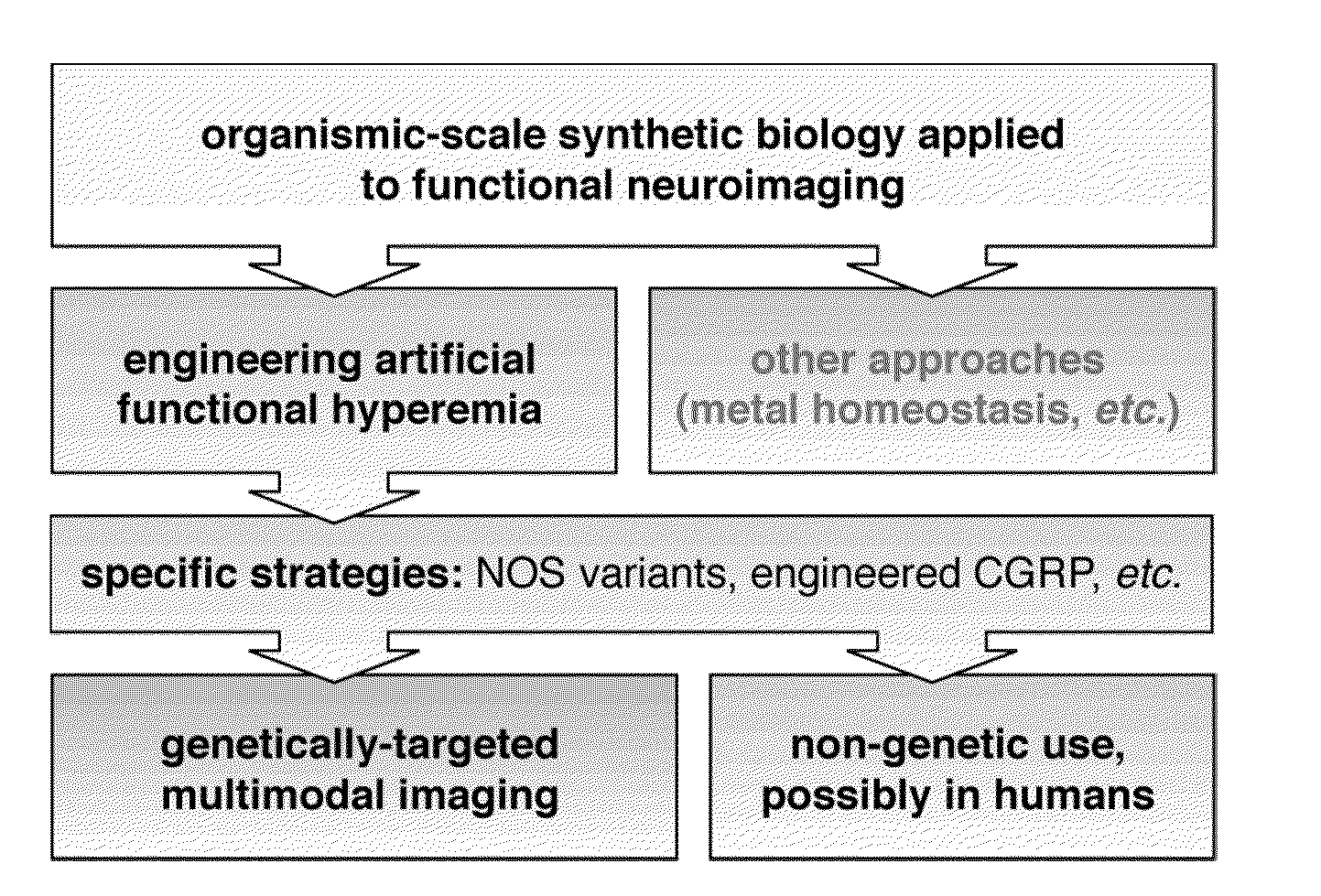
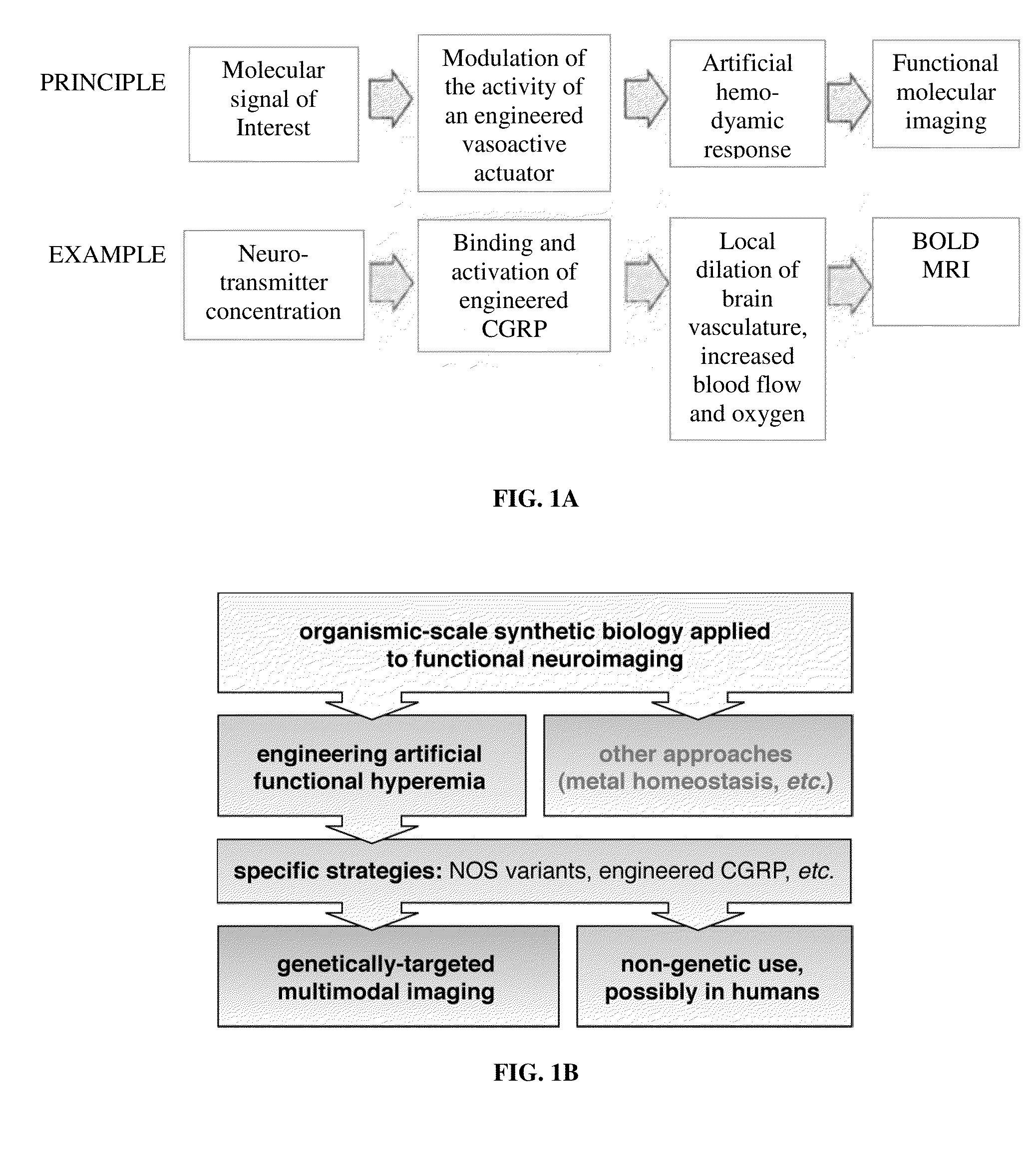
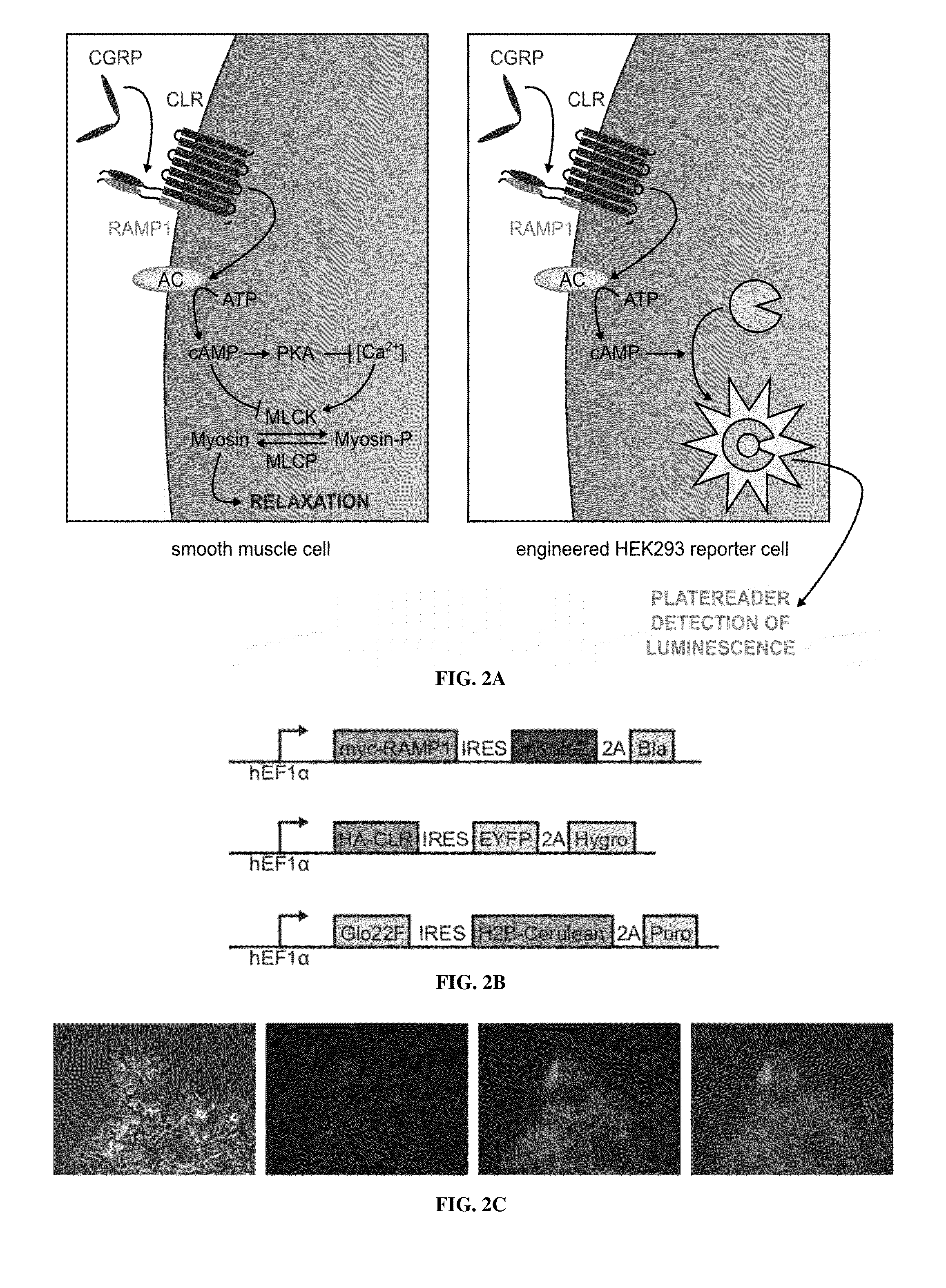
Molecular and cellular imaging using engineered hemodynamic responses
InactiveUS20150018665A1High sensitivityConfidencePeptide/protein ingredientsAntibody mimetics/scaffoldsHaemodynamic responseMolecular imaging
According to some aspects, the invention relates to methods and compositions for evaluation of hemodynamic responses (e.g., using molecular imaging) with high sensitivity.
Owner:MASSACHUSETTS INST OF TECH
Detection of neureopeptides associated with pelvic pain disorders and uses thereof
InactiveUS20080070239A1Effective treatmentRelieve symptomsOrganic active ingredientsBiocideDense Core VesiclesSensory neuron
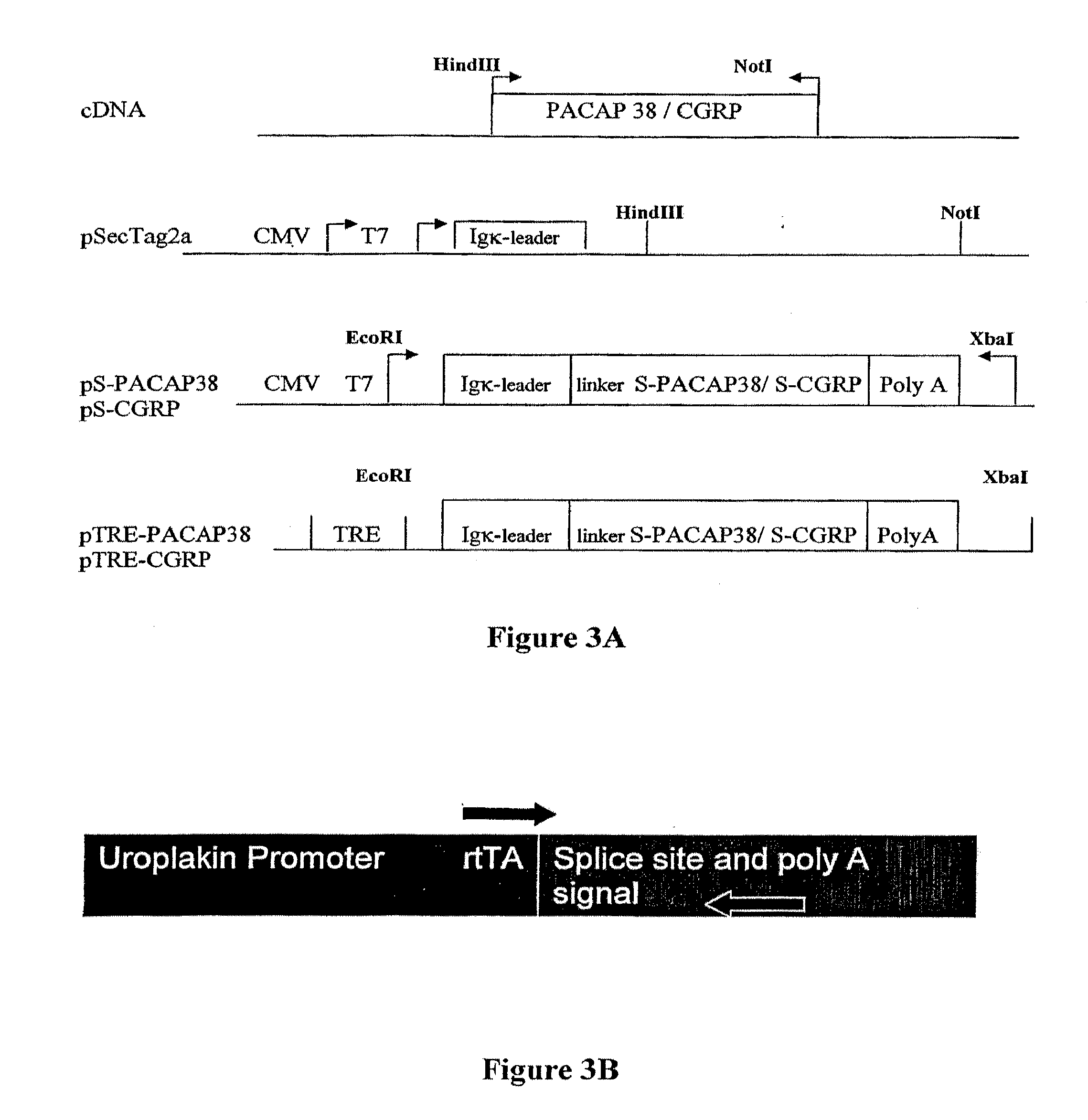
Diagnostic assessment and therapeutic treatment of pelvic pain disorders, including bladder disorders, bowel disorders, and / or reproductive tissue or organ disorders that are characterized by increased expression of the neuropeptides CGRP and / or PACAP. Additionally, applicants have developed a transgenic nonhuman model for pelvic pain disorders, where the transgenic animal expresses in bladder sensory neurons a recombinant neuropeptide implicated in the pelvic pain disorder.
Owner:UNIVERSITY OF VERMONT +1
Prognosis and risk assessment in stroke patients by determining the level of marker peptides
ActiveUS20140017808A1Increase probabilityOxytocins/vasopressinsChemiluminescene/bioluminescenceCalcitoninRisk stroke
The present invention relates to a method for prognosis of an outcome or assessing the risk of a patient having suffered a stroke or a transient ischemic attack, comprising the determination of the level of at least one marker peptide in said sample said marker peptide selected from the group comprising ANP, AVP, ADM, ET-1, troponin, CRP, calcitonin and hGH or fragments thereof or its precursor or fragments thereof and attributing the level of said at least one marker peptides its precursor or fragments thereof with the prognosis of an outcome or assessing the risk for said patient.
Owner:BRAHMS GMBH
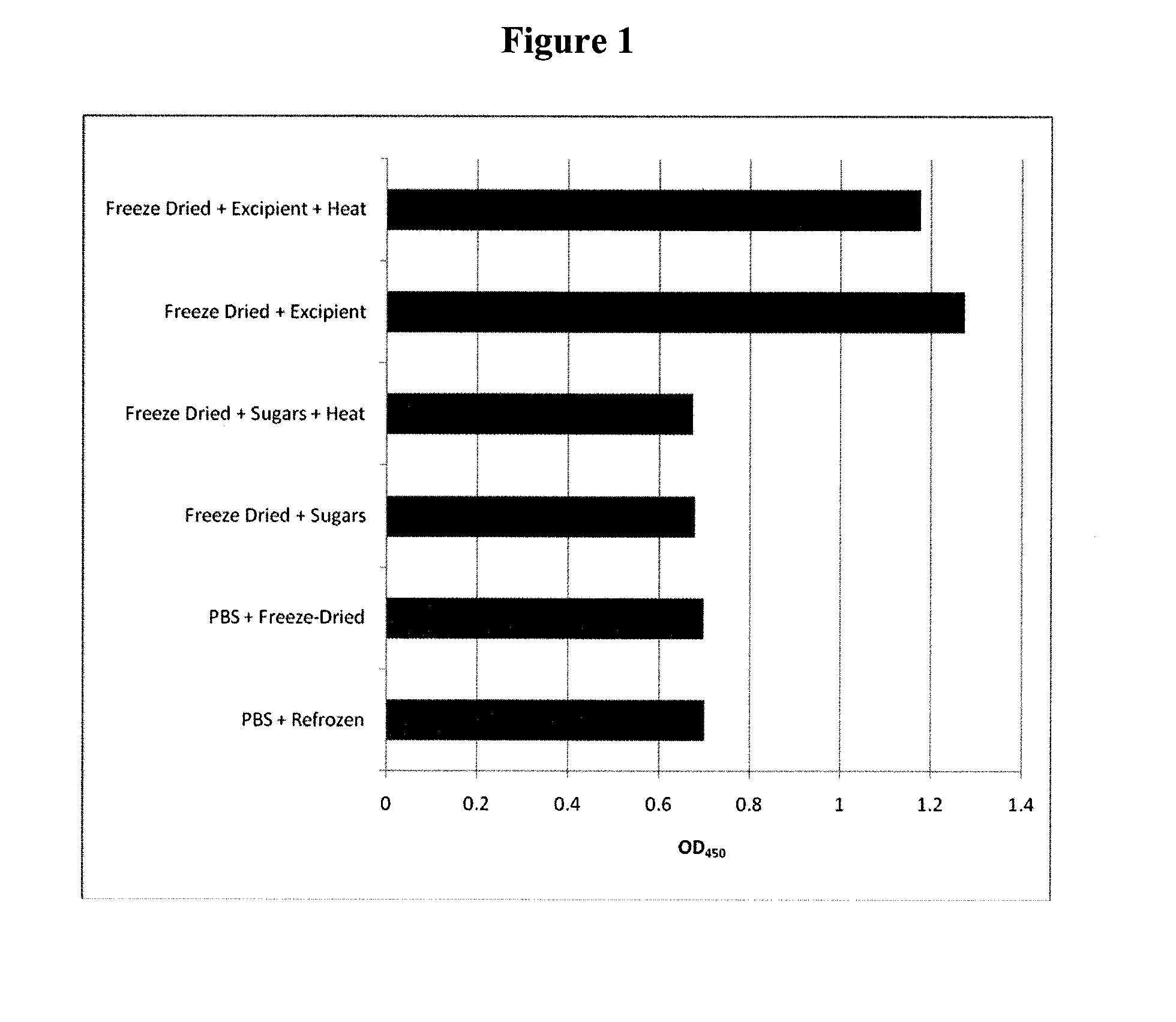
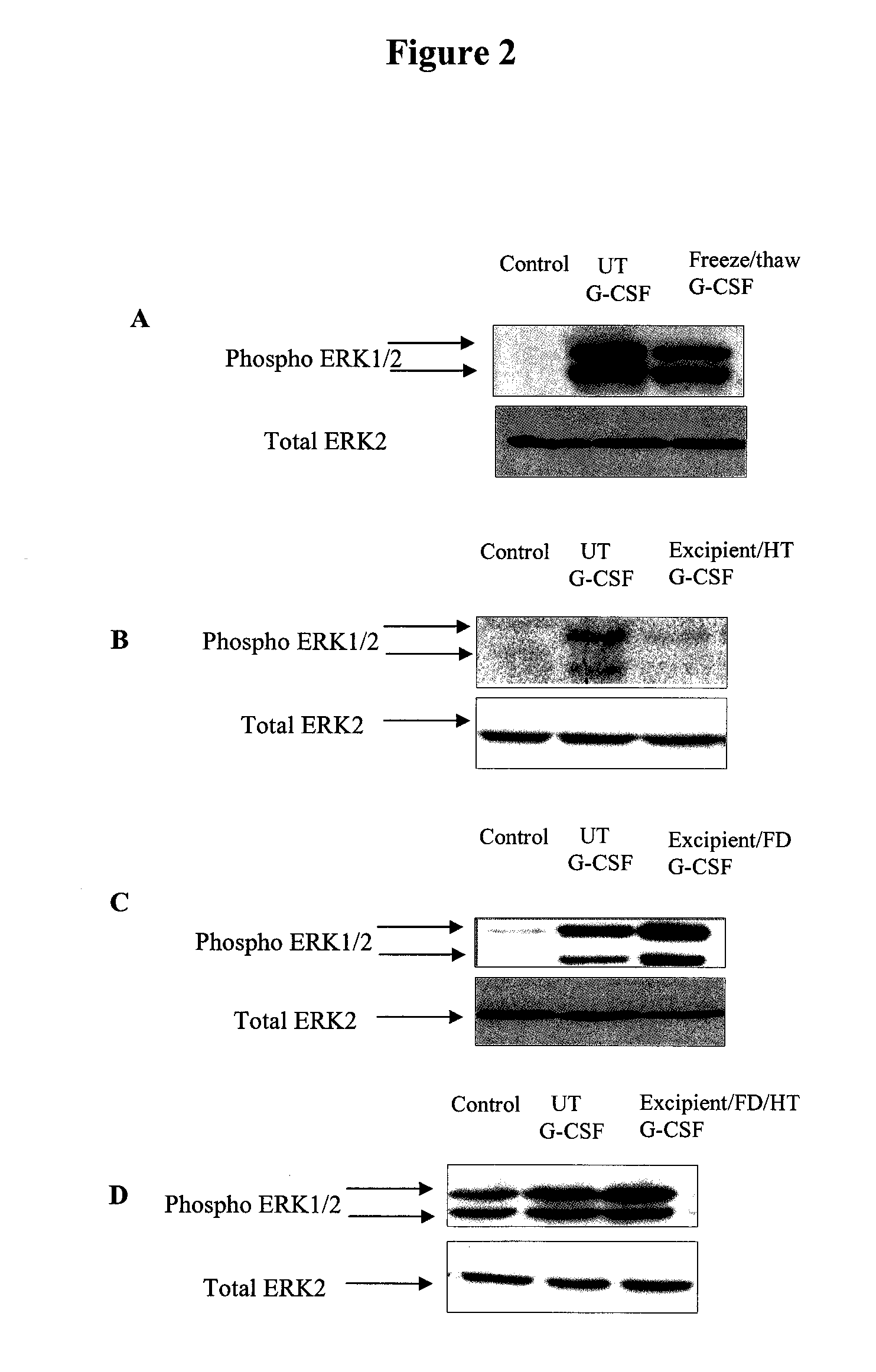
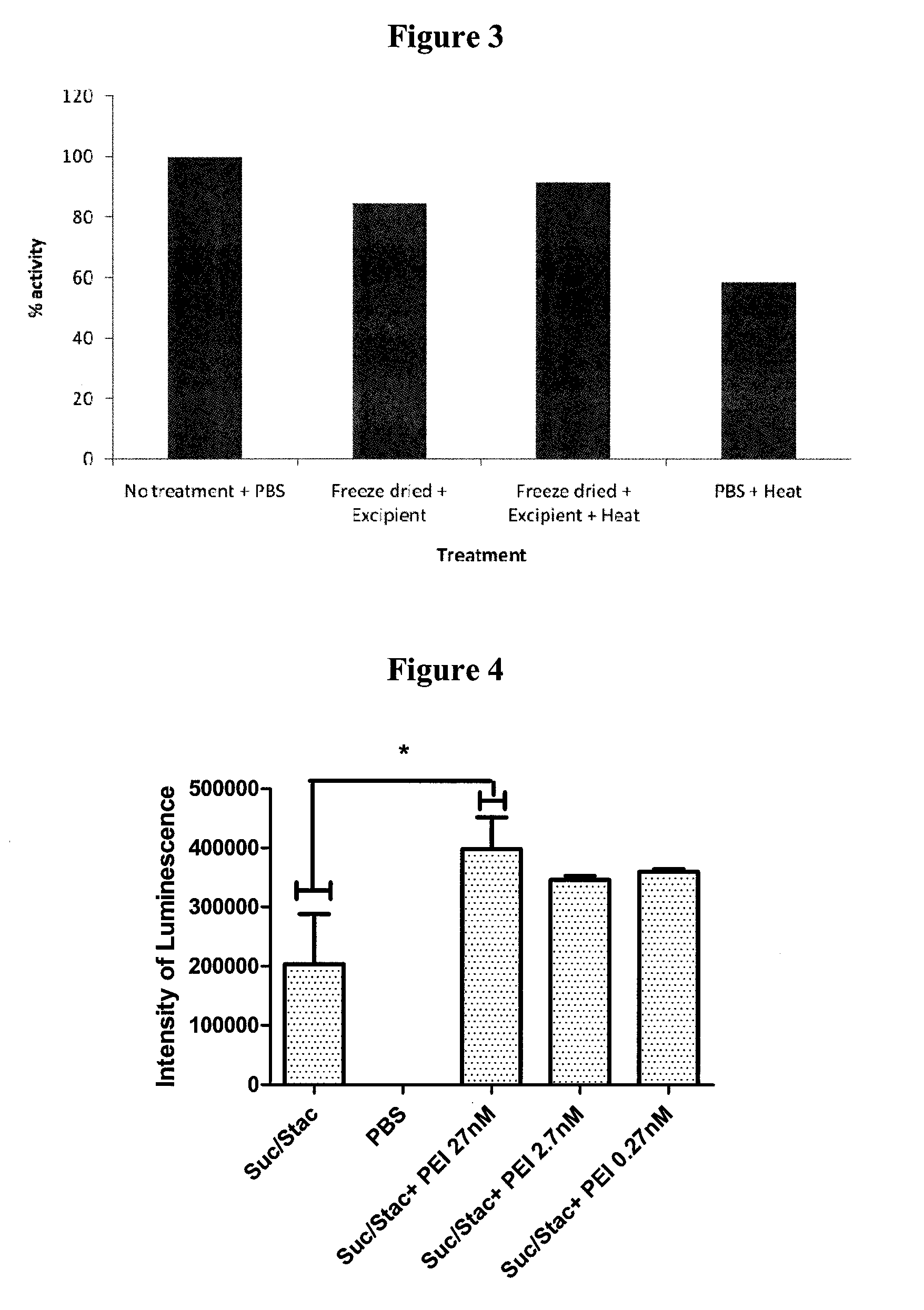
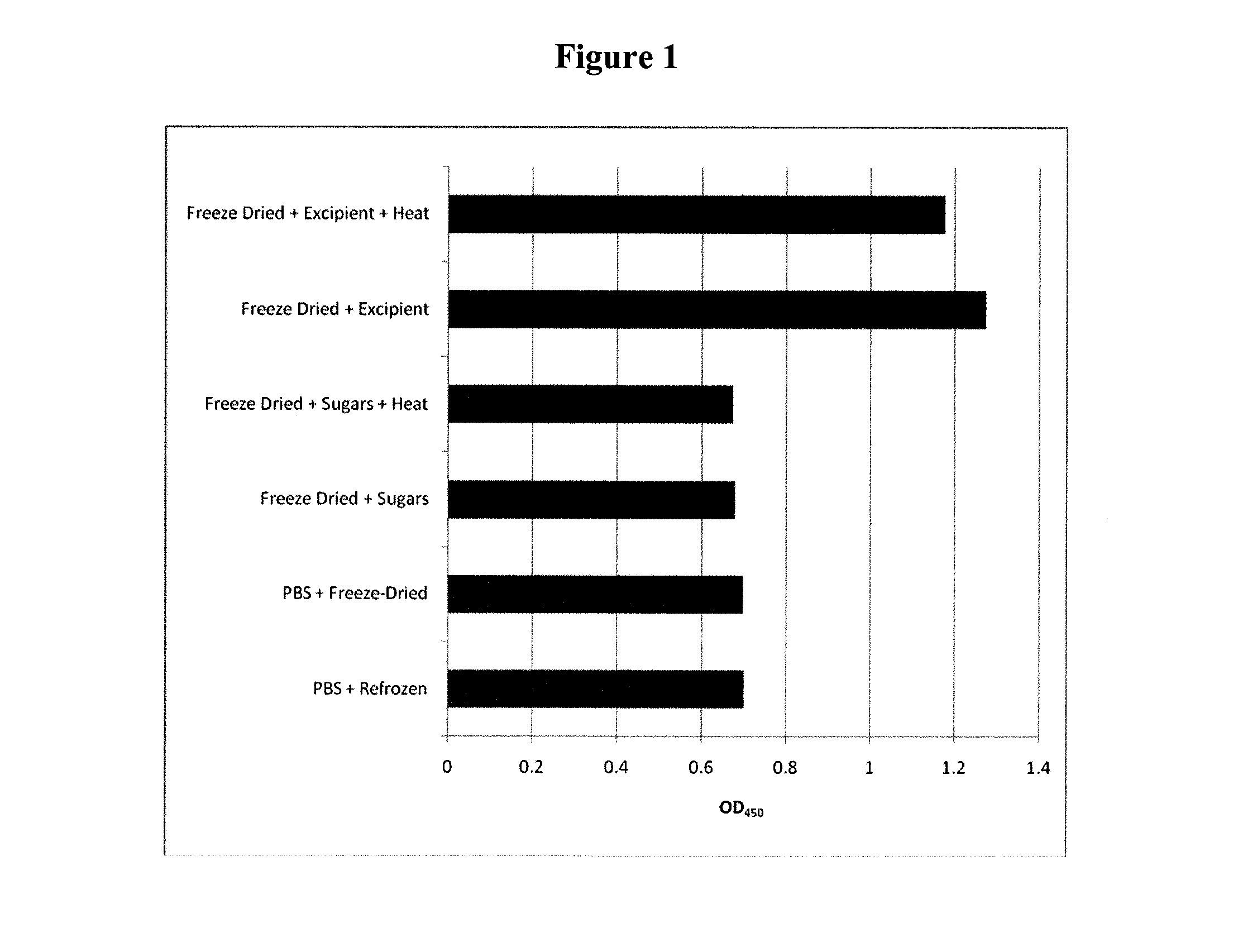
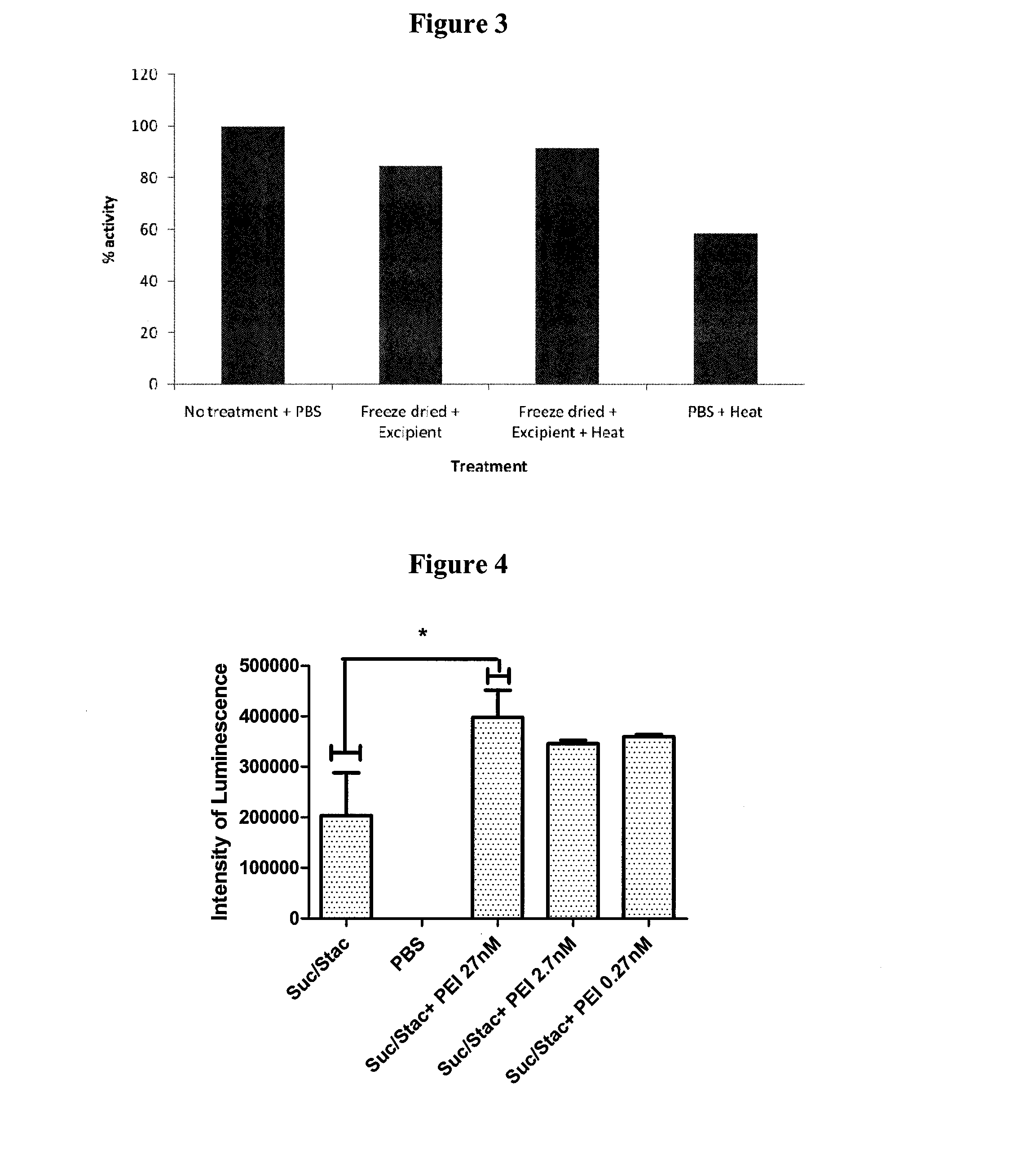
Method for Preserving Polypeptides Using a Sugar and Polyethyleneimine
The invention relates to the preservation of an active agent, such as a polypeptide, by contacting the active agent with a preservation mixture including a sugar and polyethyleneimine.
Owner:STABILITECH
Method for Preserving Polypeptides Using A Sugar and Polyethyleneimine
The invention relates to the preservation of an active agent, such as a polypeptide, by contacting the active agent with a preservation mixture including a sugar and polyethyleneimine.
Owner:STABILITECH
Detection of neuropeptides associated with pelvic pain disorders and uses thereof
InactiveUS20120058950A1Effective to treat the pelvic pain disorderRelieve symptomsBiocideOrganic active ingredientsDense Core VesiclesSensory neuron
Diagnostic assessment and therapeutic treatment of pelvic pain disorders, including bladder disorders, bowel disorders, and / or reproductive tissue or organ disorders that are characterized by increased expression of the neuropeptides CGRP and / or PACAP. Additionally, applicants have developed a transgenic non-human model for pelvic pain disorders, where the transgenic animal expresses in bladder sensory neurons a recombinant neuropeptide implicated in the pelvic pain disorder.
Owner:UNIVERSITY OF VERMONT +1
Polypeptides
ActiveUS20130059770A1Improve solubilityImprove stabilityNervous disorderPeptide/protein ingredientsPramlintideAmino acid
The invention relates to polypeptides comprising an amino acid sequence which is an analogue of pramlintide, pharmaceutical compositions comprising these polypeptides, and these polypeptides for use as medicaments.
Owner:NOVO NORDISK AS
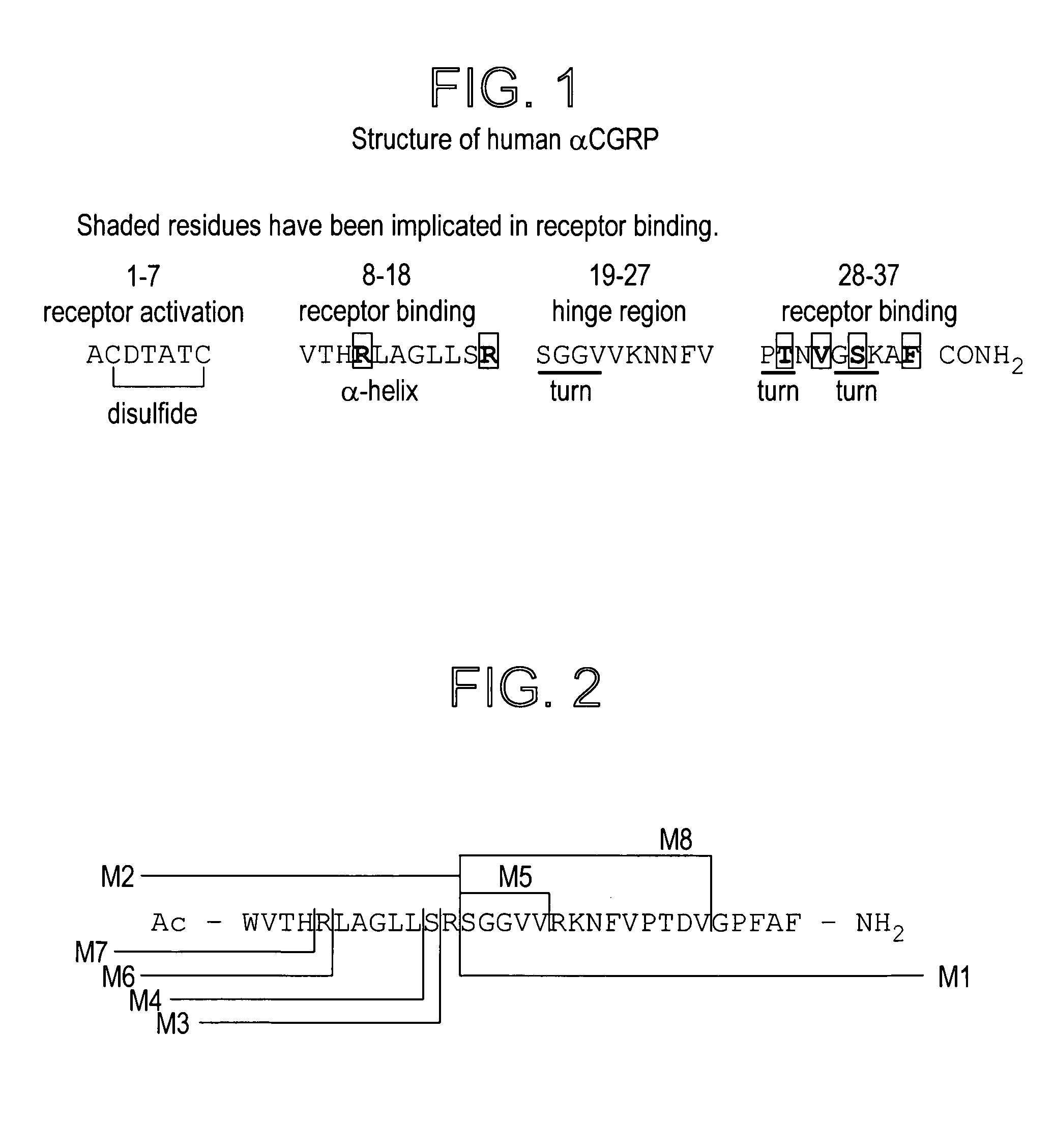
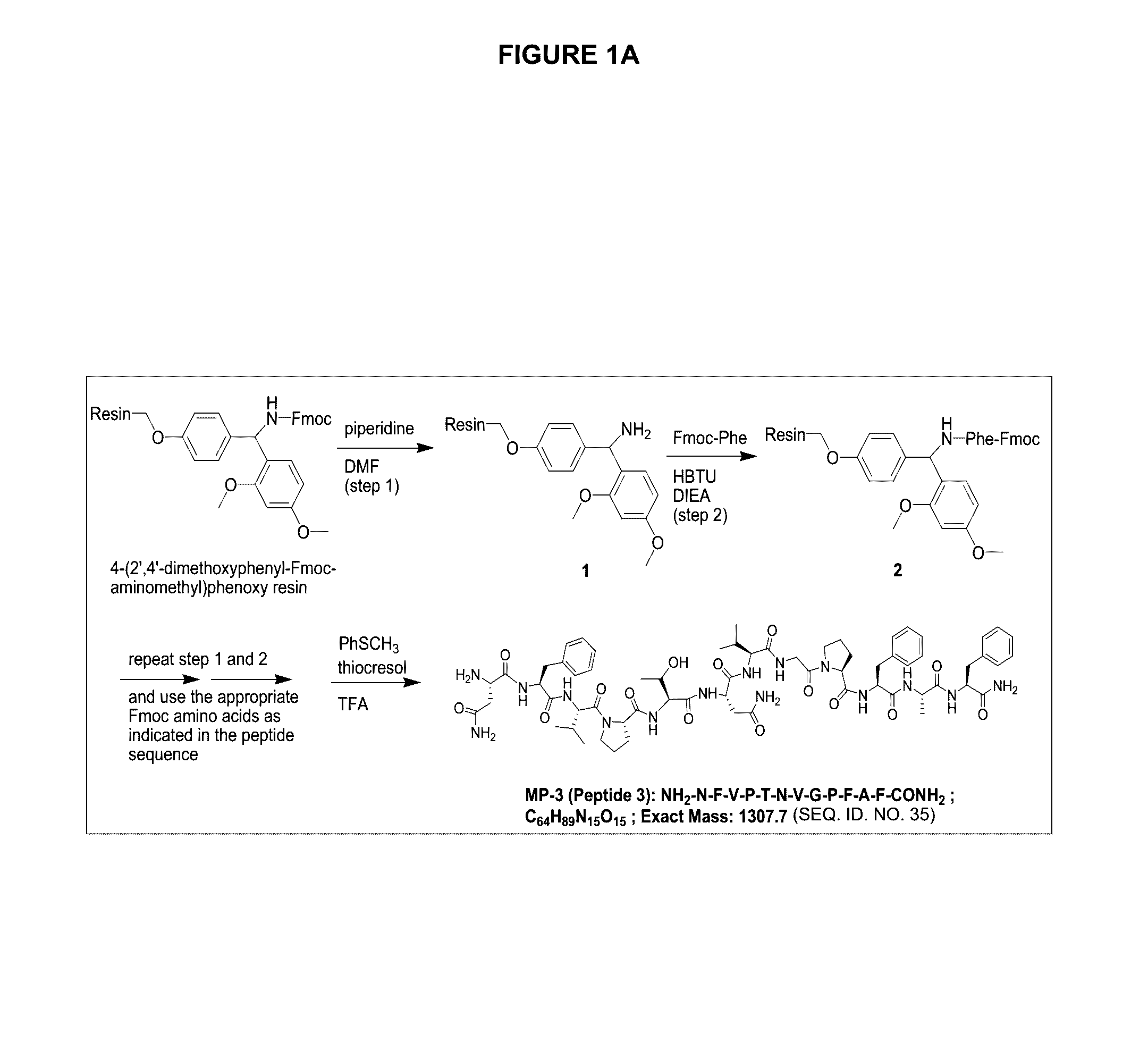
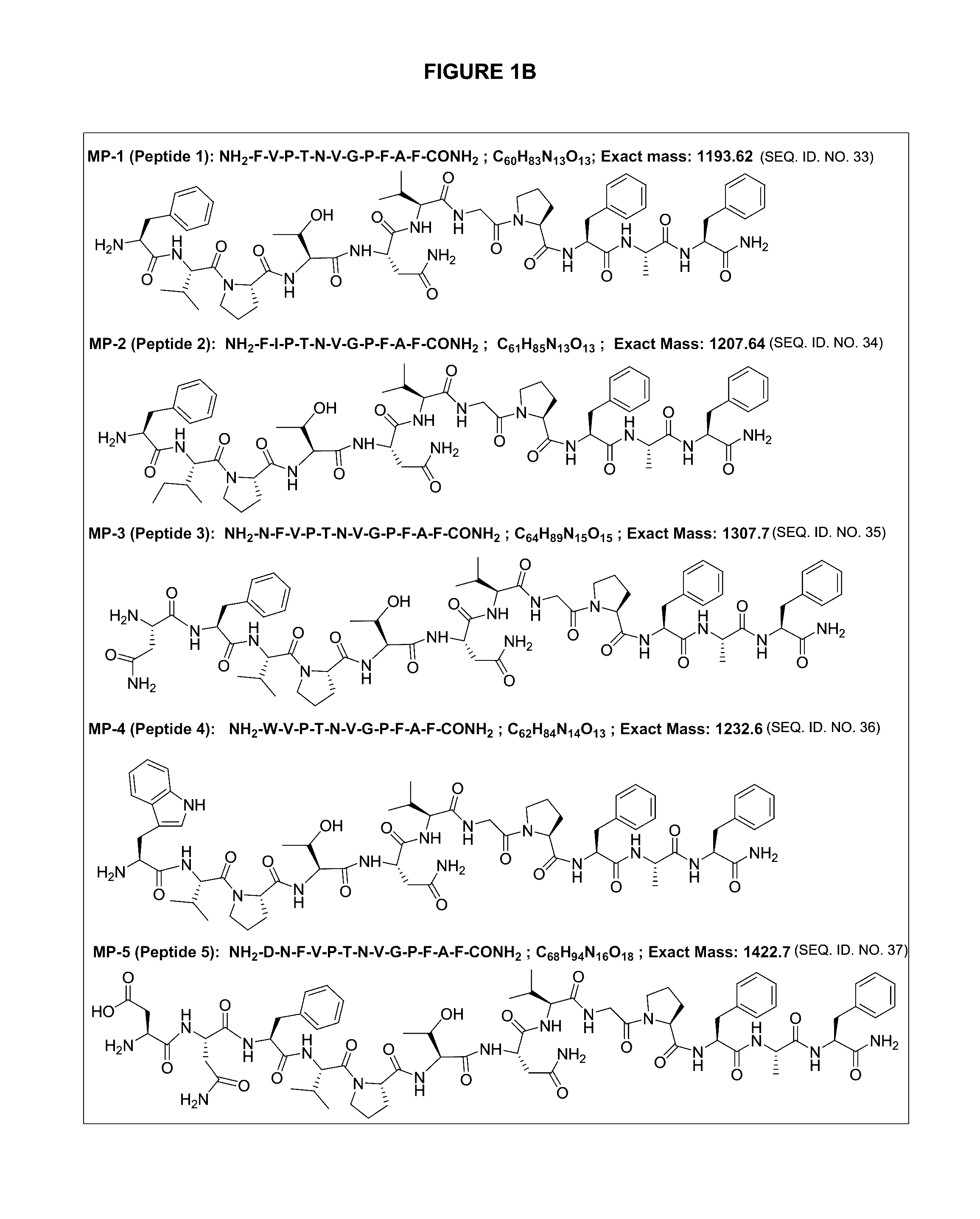
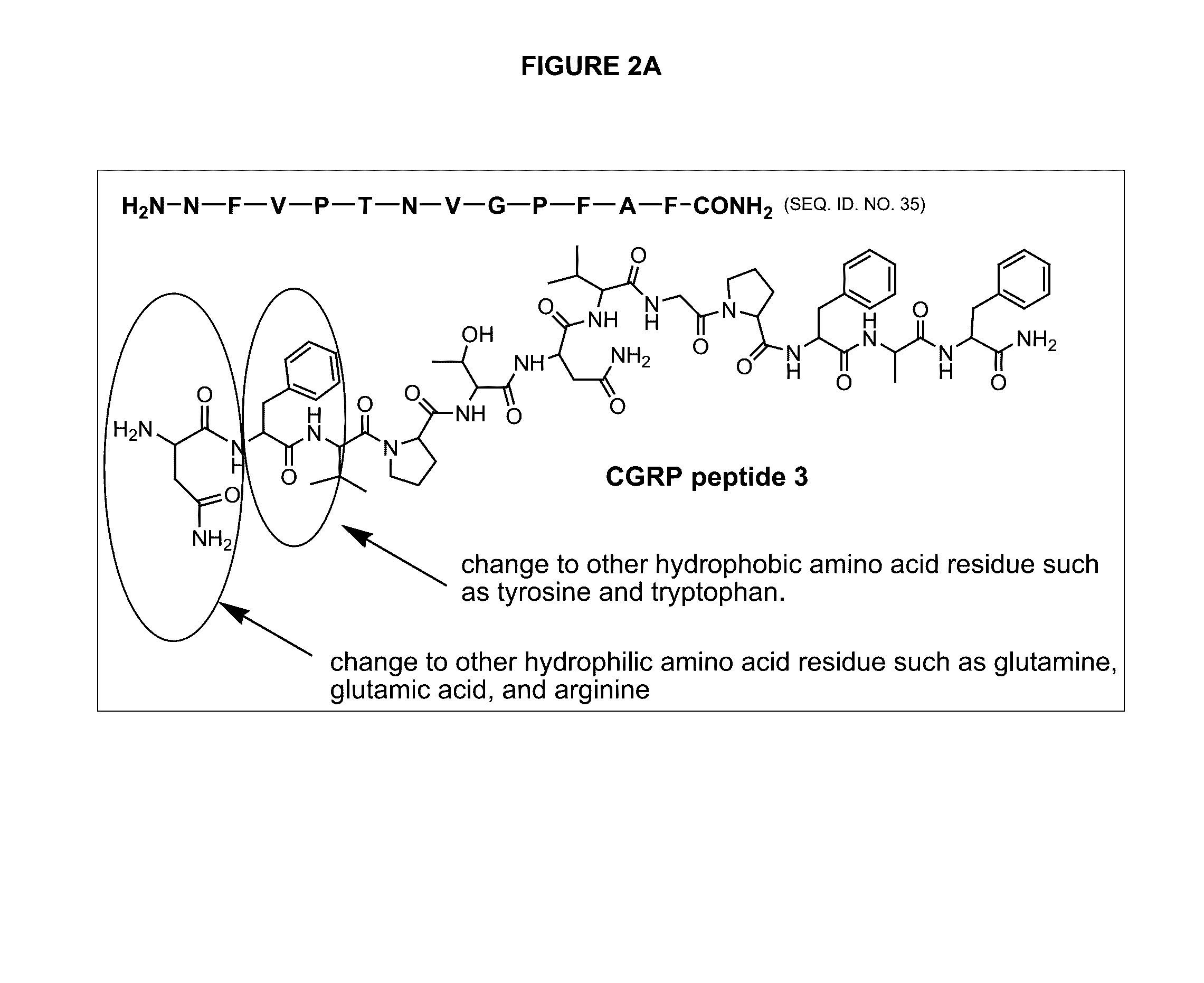
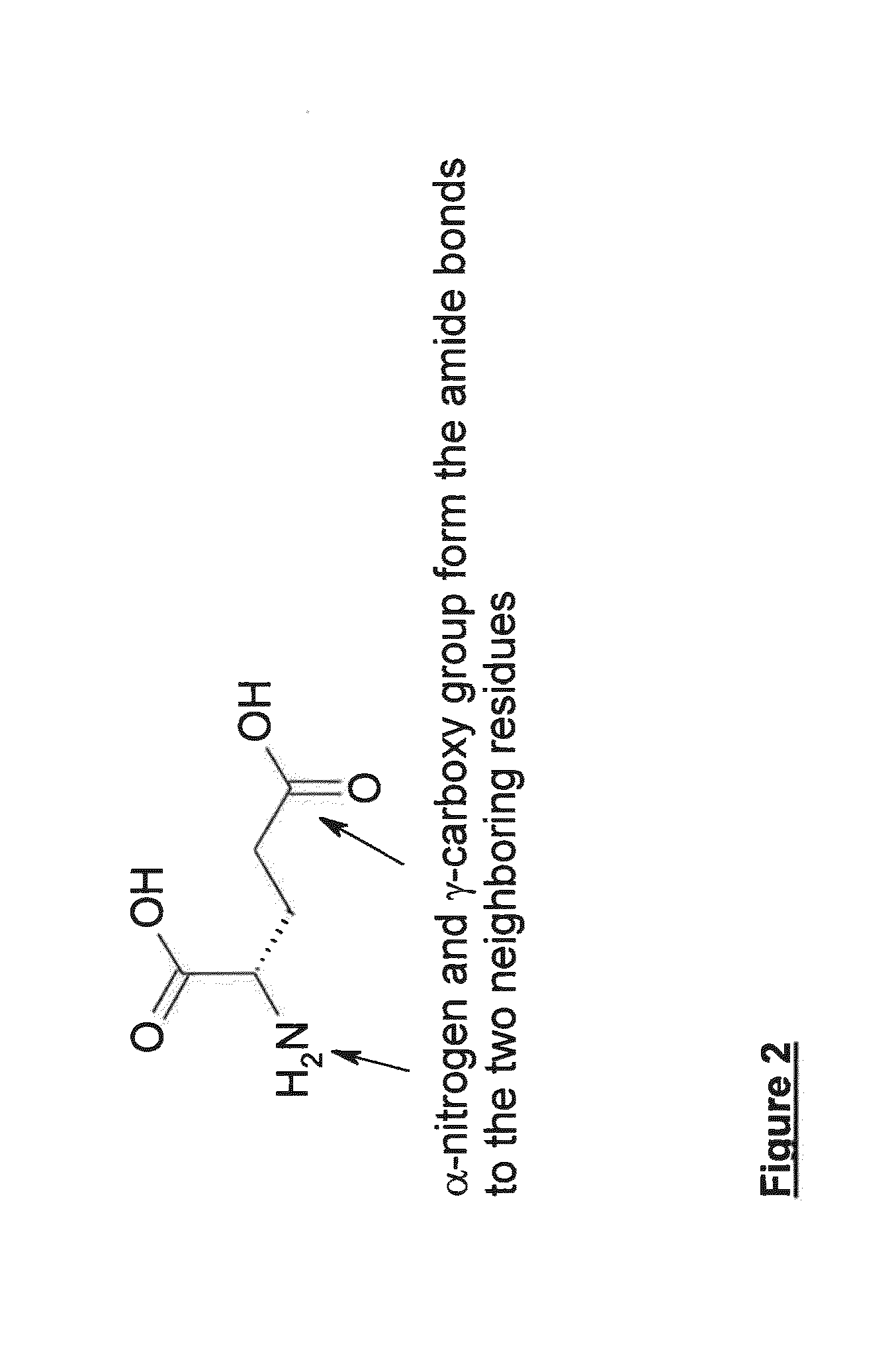
Peptide antagonists of the calcitonin cgrp family of peptide hormones and their use
The embodiments provide a modified calcitonin gene-related peptide antagonist including an N-terminal fragment of modified calcitonin gene-related peptide or related protein family member where at least two residues of the N-terminal fragment are cysteine (Cys) and at least one amino acid comprises a non-threonine substitution of a threonine (Thr) residue; a central core where the central core comprises an oc-helix; and a C-terminal fragment of modified calcitonin gene-related peptide or related protein family member comprising a C-terminal amide and where at least one amino acid of the C-terminal fragment is phenylalanine (Phe), proline (Pro), tyrosine (Tyr) or hydroxyproline (Hyp) or pharmaceutically acceptable salt thereof, as well as compositions, including pharmaceutical compositions, comprising a subject peptide. The embodiments further provide treatment methods, including methods of treating a migraine, the methods generally involving administering to an individual in need thereof an effective amount of a subject peptide or composition.
Owner:SOARES CHRISTOPHER J
Polypeptides
ActiveUS20130005646A1Improve physical stabilityIncreased solubility stabilityNervous disorderPeptide/protein ingredientsAmino acidAcid amino sequences
The invention relates to polypeptides comprising an amino acid sequence which is an analogue of pramlintide, pharmaceutical compositions comprising these polypeptides, and these polypeptides for use as medicaments.
Owner:NOVO NORDISK AS
Peptide antagonists of the calcitonin CGRP family of peptide hormones and their use
The embodiments provide a modified calcitonin gene-related peptide antagonist including an N-terminal fragment of modified calcitonin gene-related peptide or related protein family member where at least two residues of the N-terminal fragment are cysteine (Cys) and at least one amino acid comprises a non-threonine substitution of a threonine (Thr) residue; a central core where the central core comprises an α-helix; and a C-terminal fragment of modified calcitonin gene-related peptide or related protein family member comprising a C-terminal amide and where at least one amino acid of the C-terminal fragment is phenylalanine (Phe), proline (Pro), tyrosine (Tyr) or hydroxyproline (Hyp) or pharmaceutically acceptable salt thereof, as well as compositions, including pharmaceutical compositions, comprising a subject peptide. The embodiments further provide treatment methods, including methods of treating a migraine, the methods generally involving administering to an individual in need thereof an effective amount of a subject peptide or composition.
Owner:SOARES CHRISTOPHER J
Intermedin related polypeptide and application thereof in preventing and treating sepsis
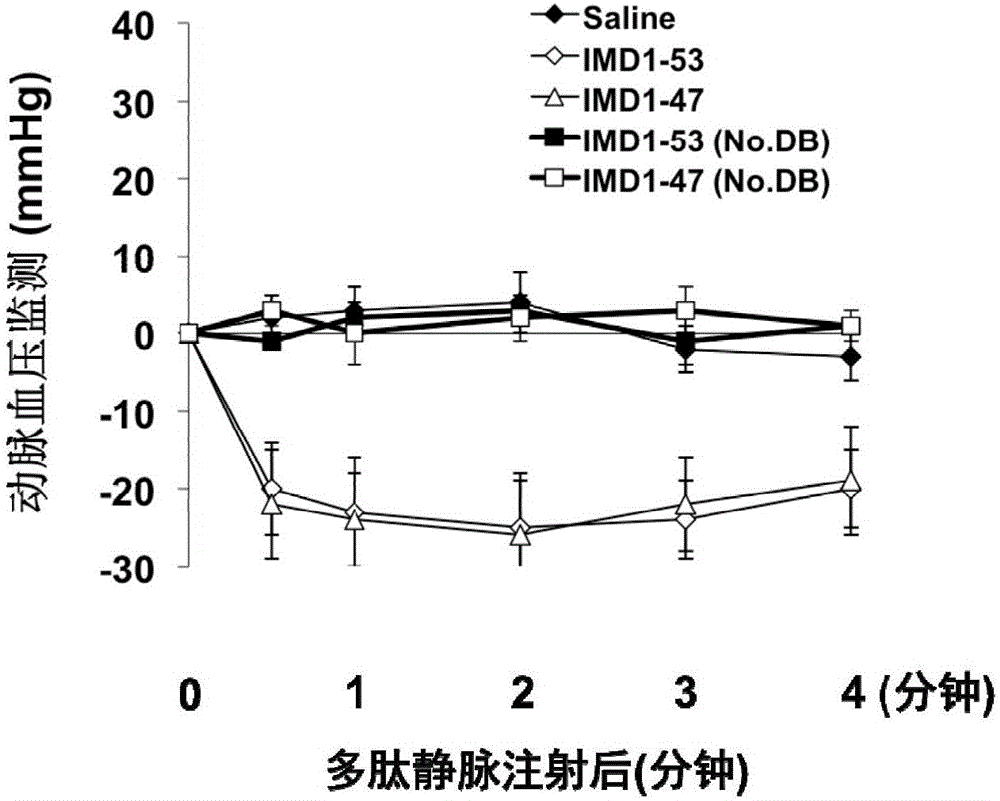
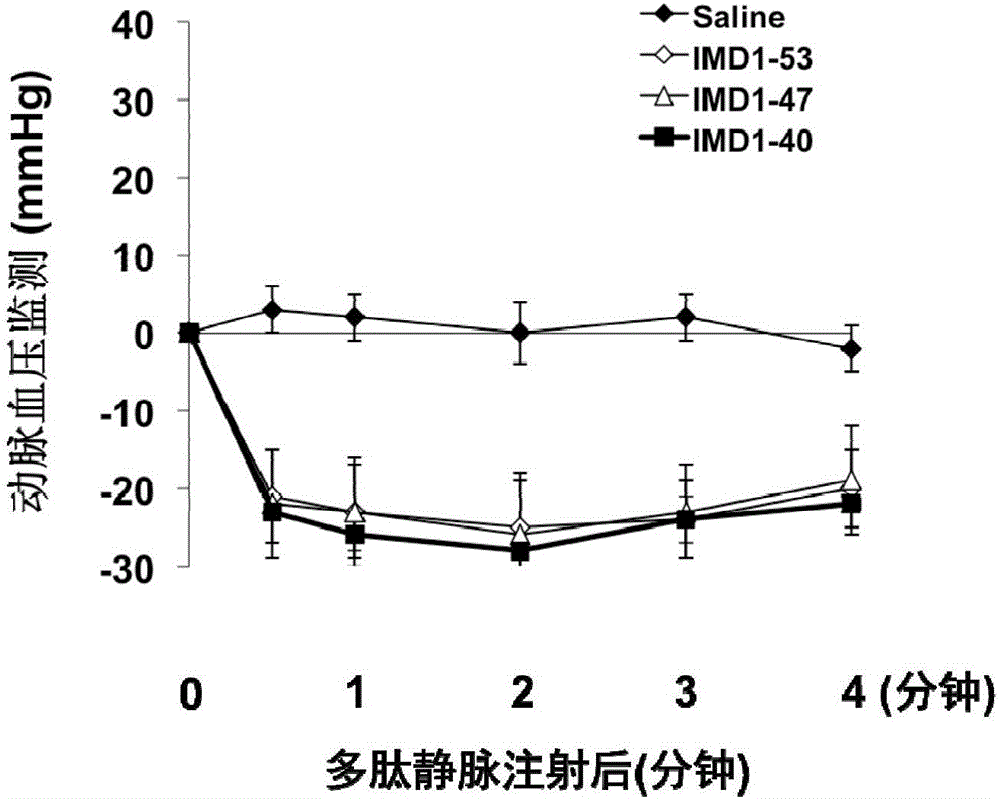
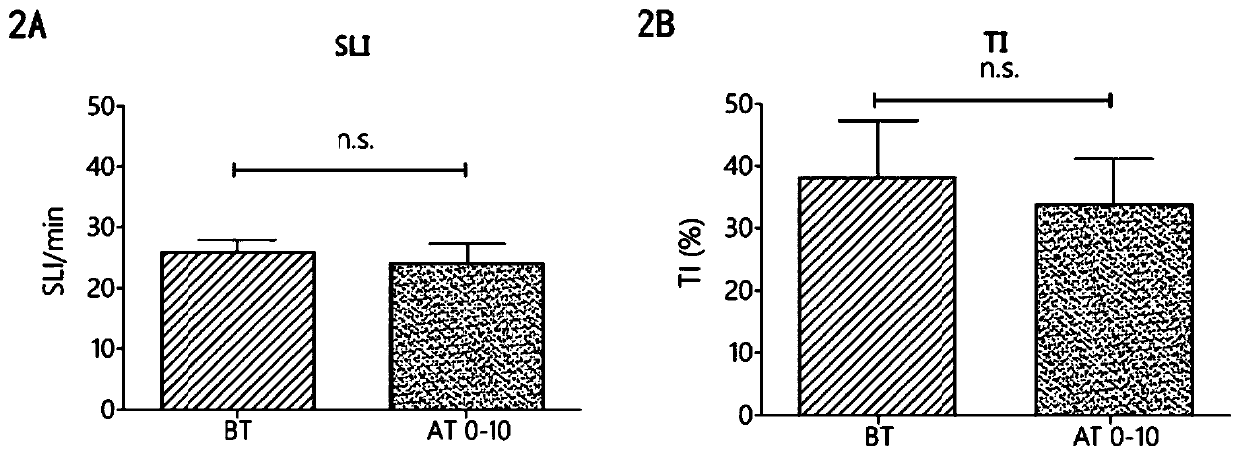
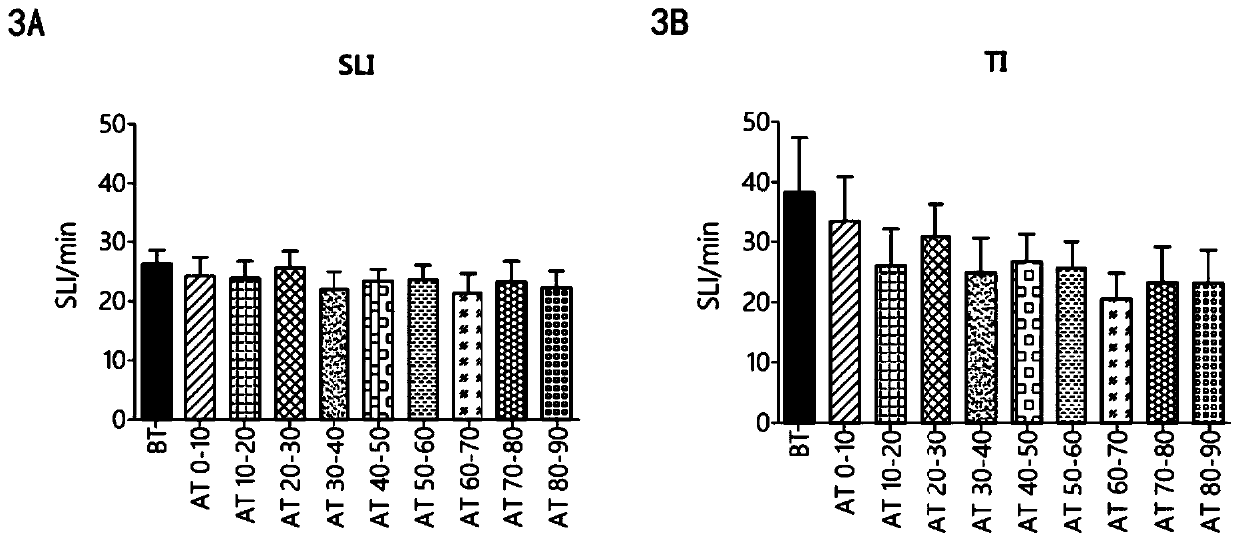
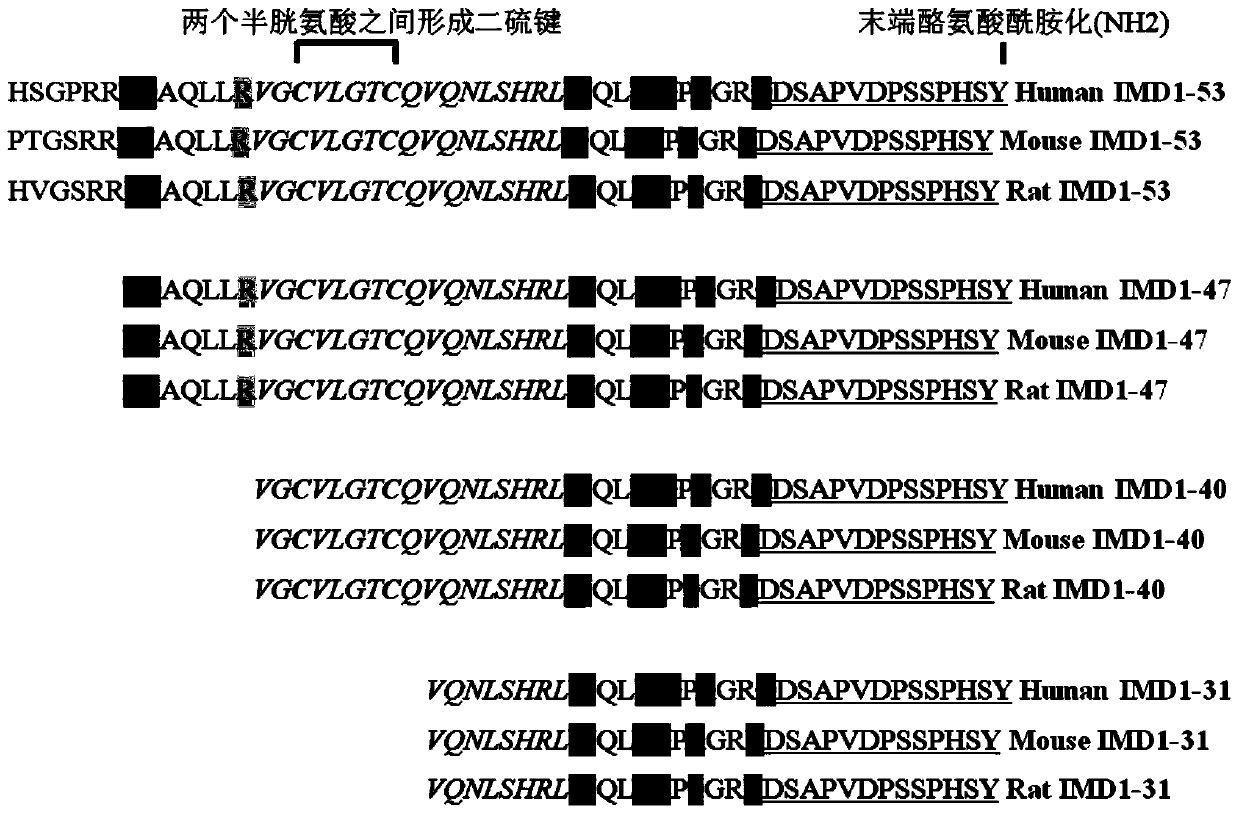
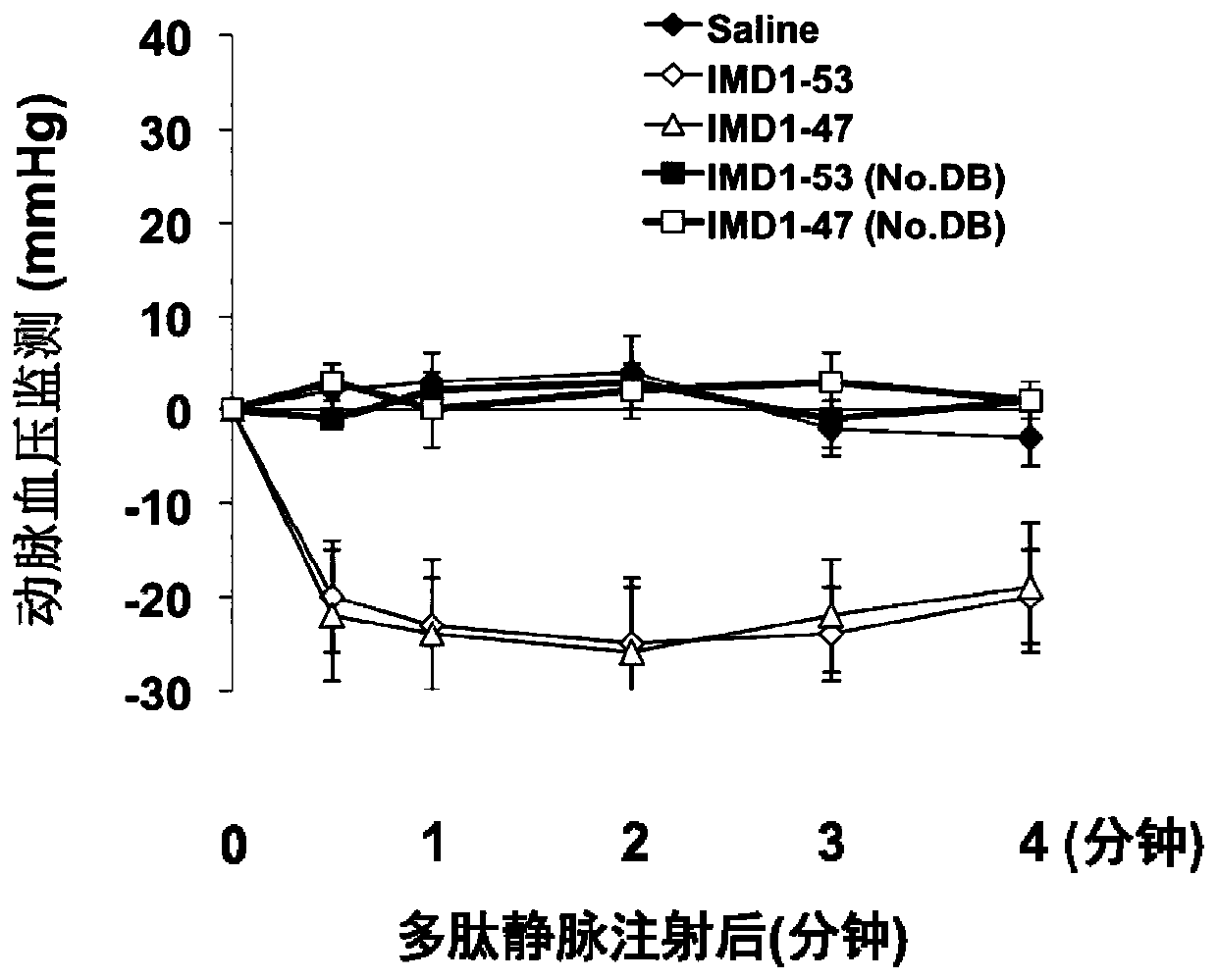
ActiveCN106749612AReduce leakageReduce edemaAntibacterial agentsPeptide/protein ingredientsVascular endotheliumAdrenomedullin 2
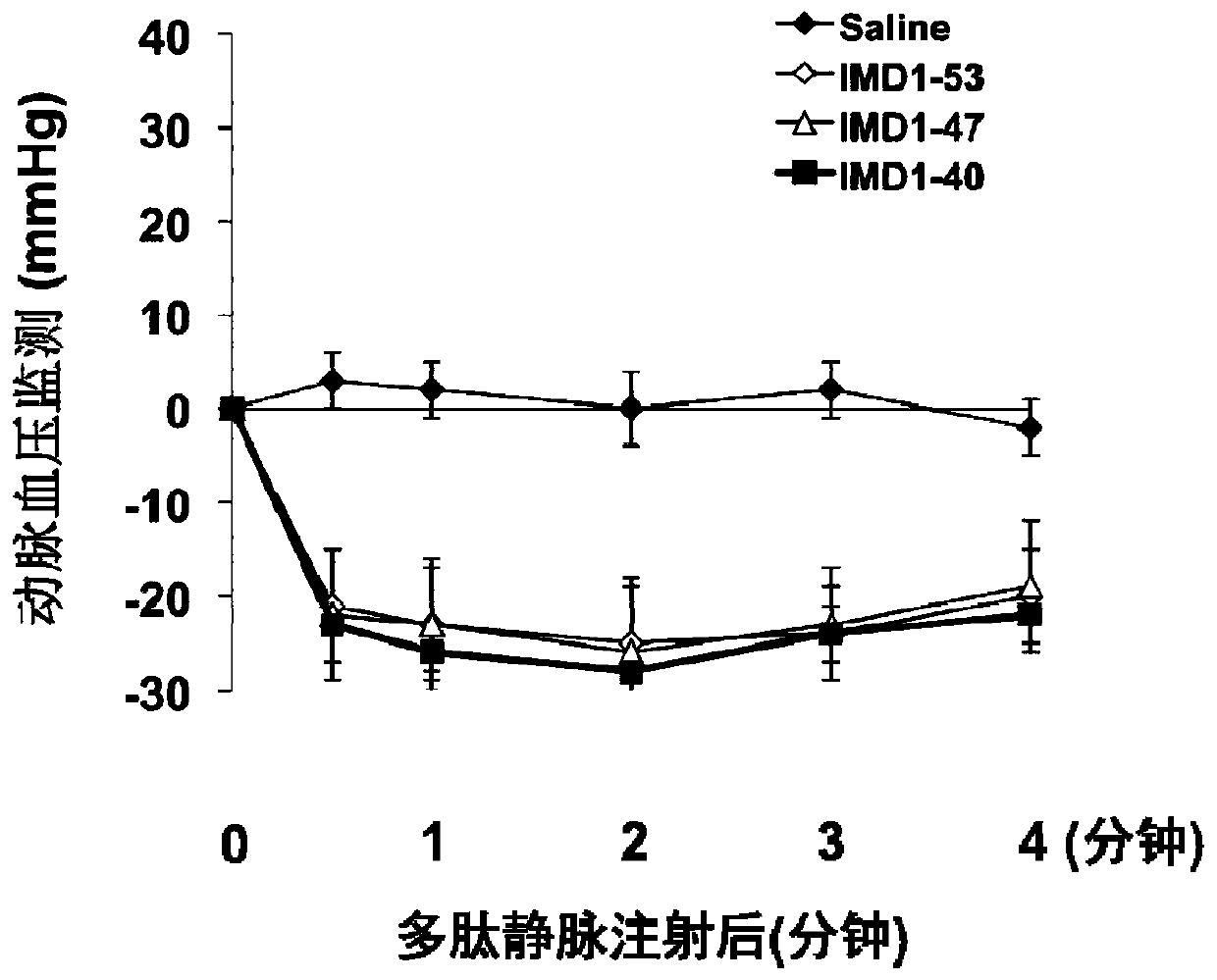
The invention belongs to the field of biological medicine and relates to polypeptide fragment derived from intermedin (IMD; alternate name, Adrenomedullin 2, ADM2). The polypeptide fragment is specifically mature peptide IMD108-147 with the length of 40 amino acids. Researches show that intermedin precursor peptide and three kinds of sheared manure peptides can reduce cytokine storm caused by sepsis, have a protection effect on vascular endothelial barriers, can reduce tissue edema and organic damage, can effectively reduce death rate of the sepsis. The IMD108-147 related in the invention has possibility of serving as polypeptide medicine to be applied to preventing and treating clinical sepsis and has a very good application prospect.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
Compositions and methods for treating acute and chronic pain by local antagonism of cgrp receptors, or combination with sodium channel inhibition or with Anti-inflammatory agents
ActiveUS20160347810A1Decreasing neurogenic inflammationReduced undesirable systemic side effectPeptide/protein ingredientsPharmaceutical delivery mechanismSodium Channel InhibitorsSide effect
The present invention provides compositions, and methods for local administration of certain peptides or combination with certain small molecules that produce analgesia and anti-inflammation in a mammal. Exemplary polypeptides provide peripheral analgesia and anti-inflammation when administered via local topical, subcutaneous, intradermal, or intranasal administration, to provide analgesia and anti-inflammation. Through antagonism of peripheral CGRP receptors alone, or in combination with inhibition of sensory sodium channels or anti-inflammation, the compositions of the invention provide local therapeutic pain relief with minimal undesired systemic side effects in a subject. Also provided are improved peptide delivery techniques including microneedle unit dose administering apparatus and methods. Also provided are hydrogel formulations for sustained local delivery to a subject of one or more of the compositions according to the invention in a therapeutically effective amount, thereby providing local pain relief and / or reducing associated inflammation.
Owner:AFASCI
Peptide analogs
InactiveUS20190153059A1Prevent unwanted side effectPeptide/protein ingredientsAntibody mimetics/scaffoldsG protein-coupled receptorReceptor regulator activity
Analogs for CLR / RAMP receptor ligands are provided that have agonist, superagonist, antagonist, super-antagonist, or multiple receptor modulatng activity. The analogs can be selective for one or more CLR / RAMP receptors, or can be pan-specific for multiple G protein-coupled receptors.
Owner:ADEPTHERA LLC
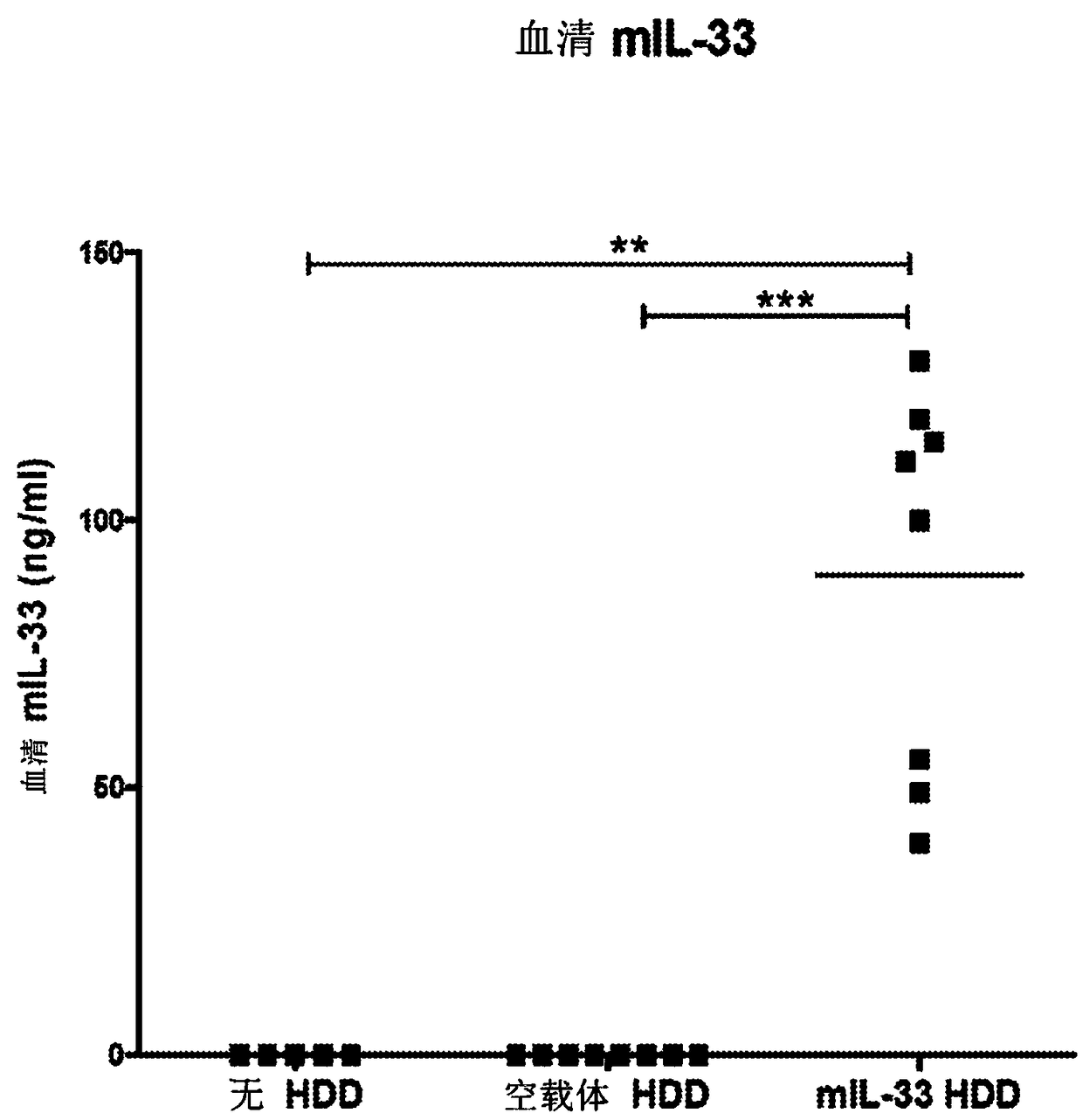
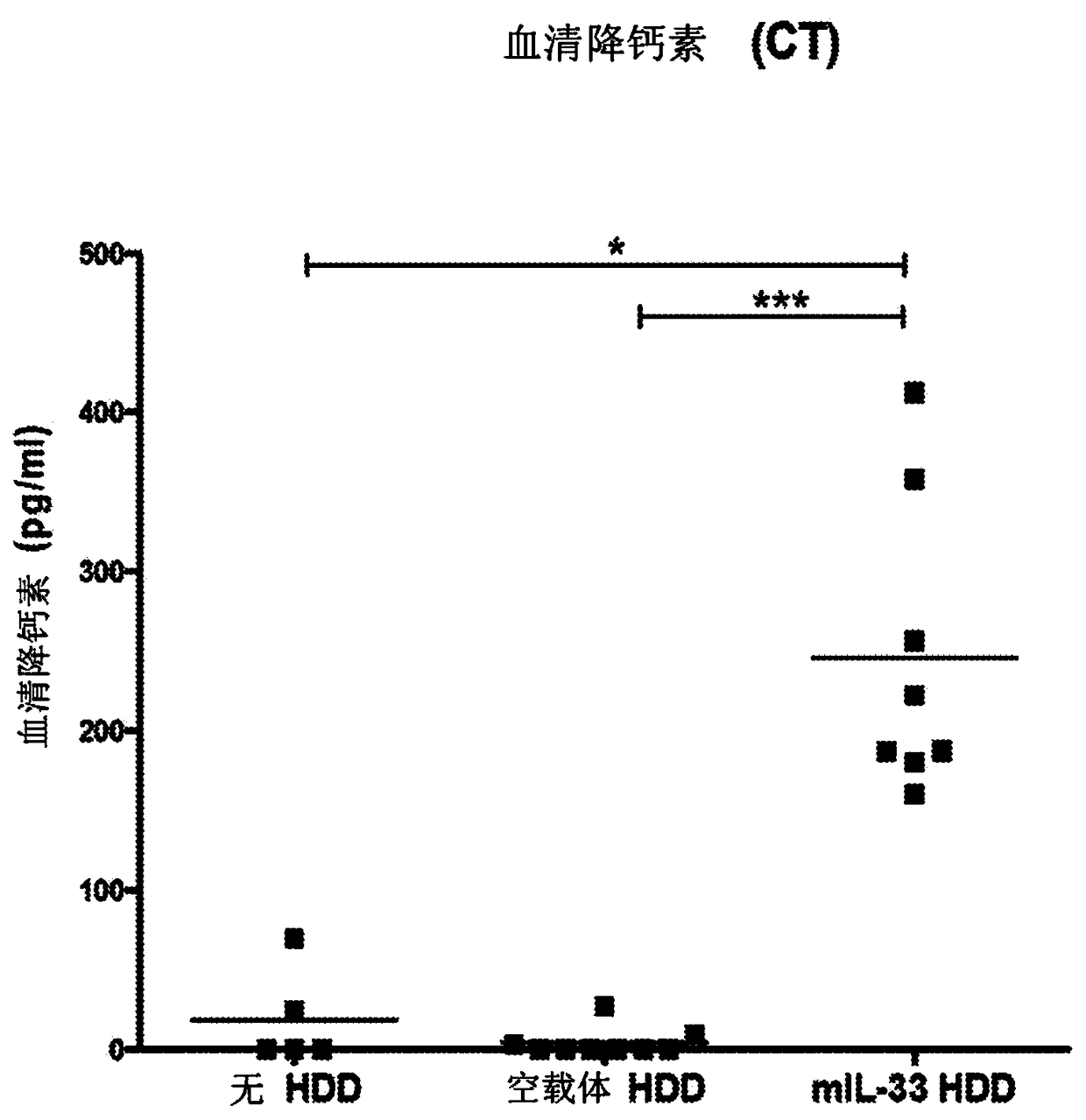
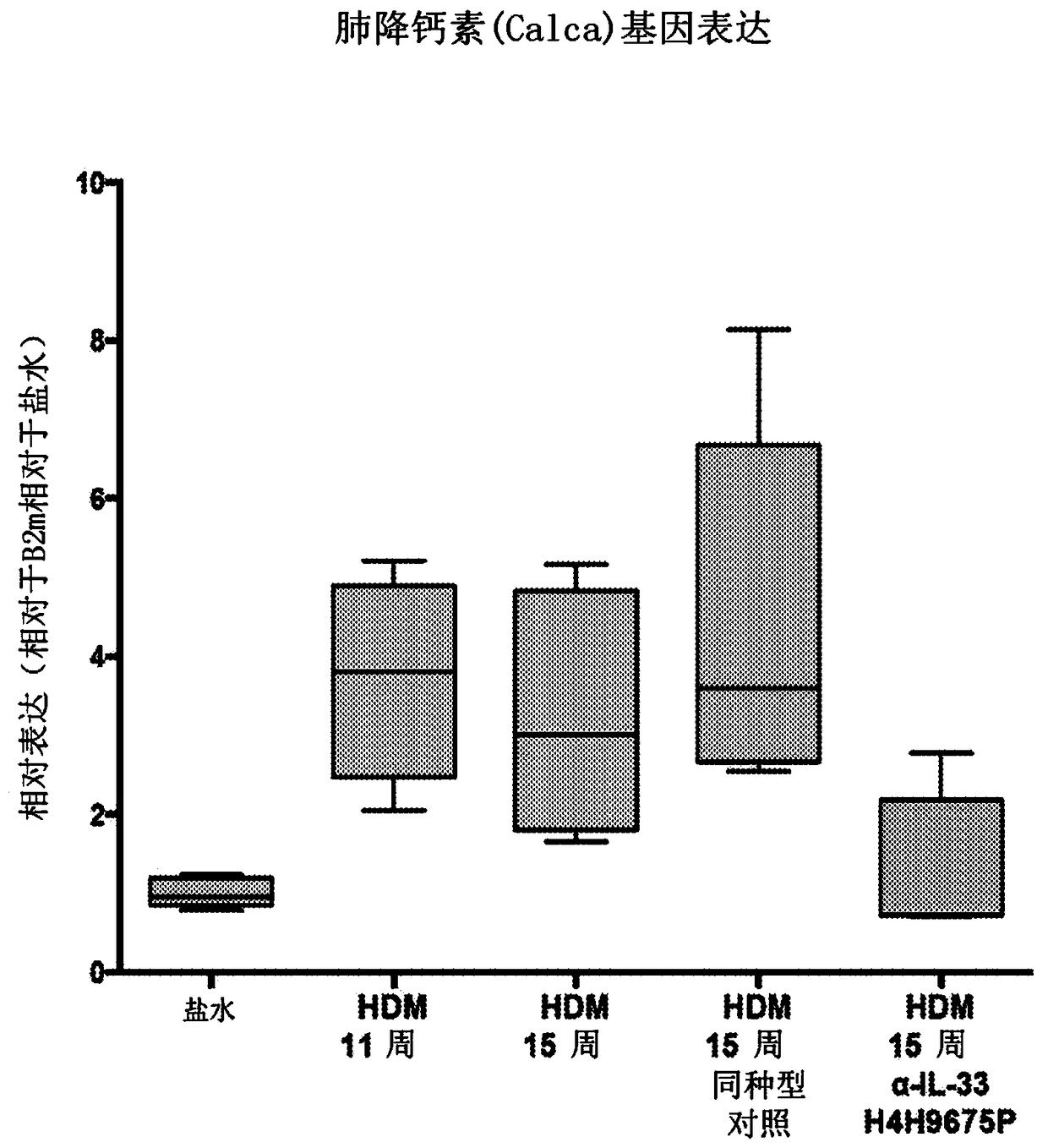
Biomarkers related to interleukin-33 (il-33)-mediated diseases and uses thereof
The present invention relates to the identification of certain biomarkers for use in identifying patients who have, or are likely to develop an IL-33 mediated disease or disorder and who are more likely to respond to therapy with an IL-33 antagonist. The invention also relates to methods of treatment of an IL-33-mediated disease or disorder in a patient by administering an IL-33 antagonist to thepatient in need thereof and monitoring the effectiveness of therapy using the biomarkers described herein. Also provided are methods for decreasing the level of at least one biomarker in a subject suffering from an IL-33-mediated disease or disorder, and methods for treating such diseases or disorders according to the expression levels of one or more biomarkers. The methods of the present invention comprise administering to a subject in need thereof a pharmaceutical composition comprising an interleukin-33 antagonist.
Owner:REGENERON PHARM INC
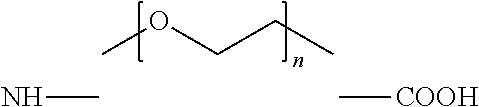
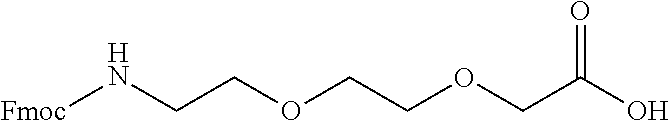
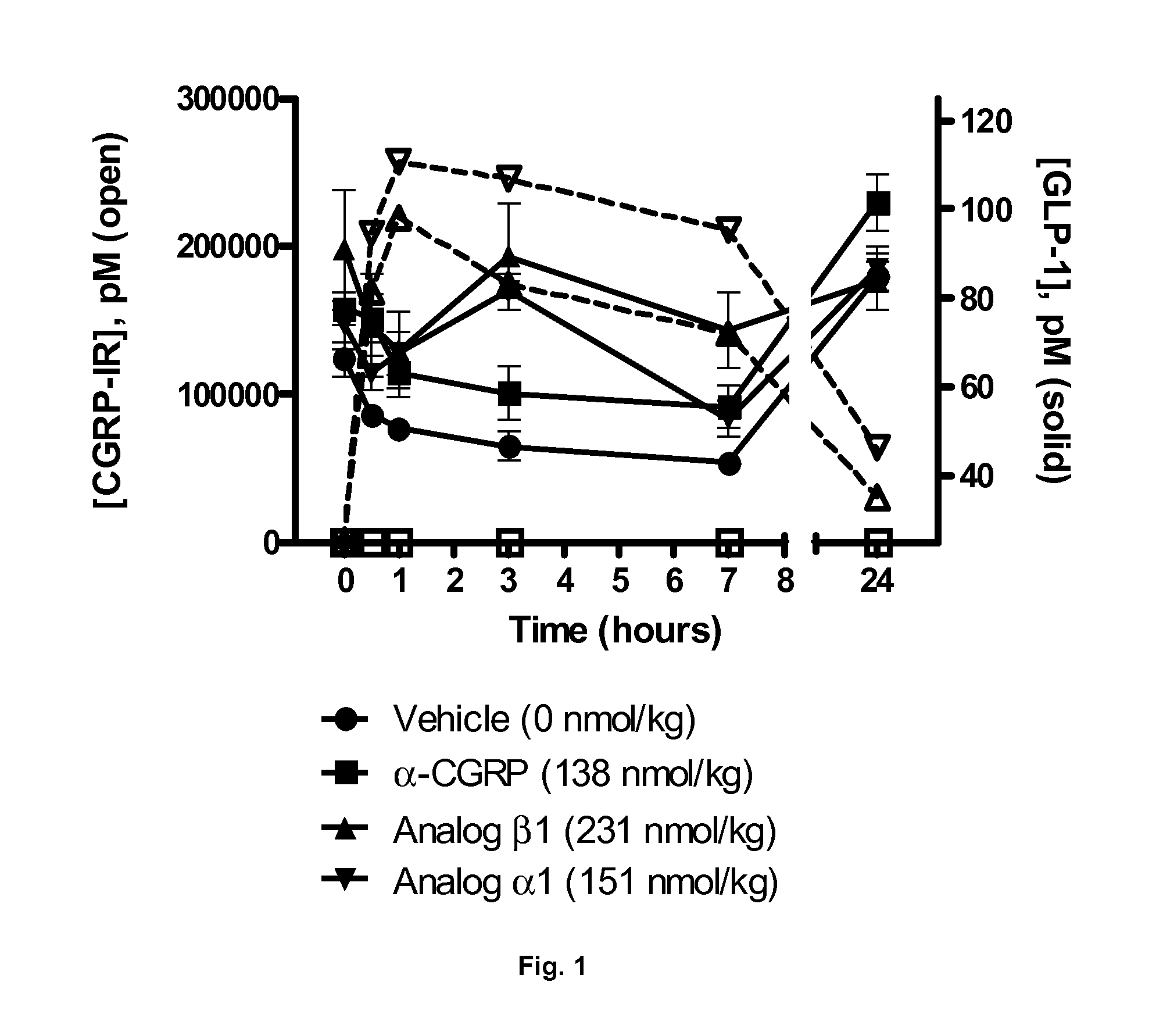
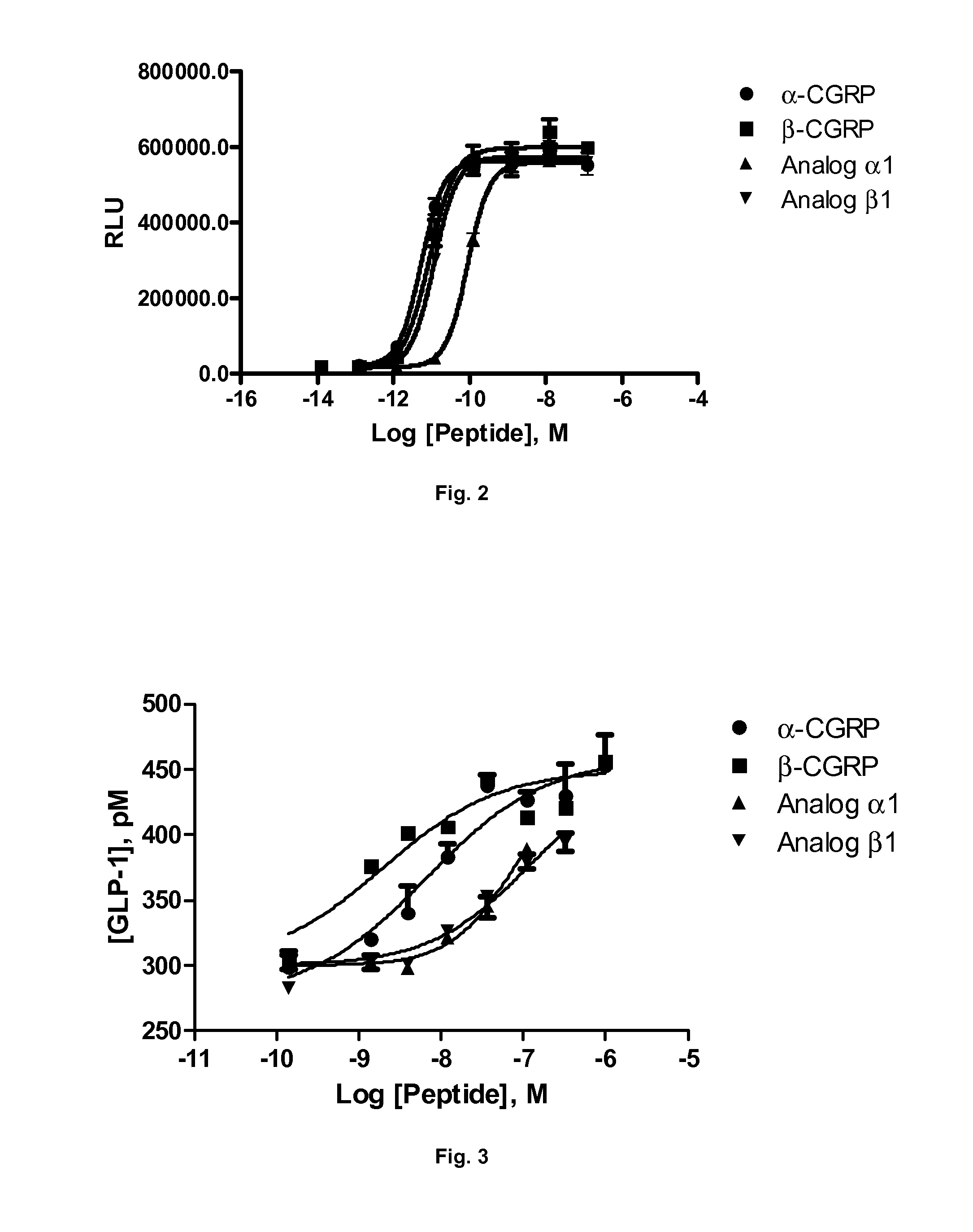
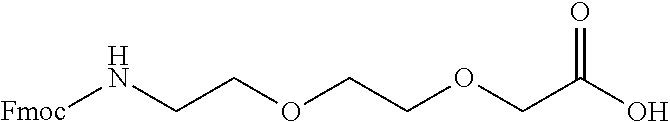
Derivatives of CGRP
ActiveUS20120245083A1Lower blood pressureImprove the level ofPeptide/protein ingredientsMetabolism disorderOrganic chemistryProlonged action
Owner:EPOQE PHARMA APS
Derivatives of CGRP
ActiveUS8835379B2Lower blood pressureImprove the level ofPeptide/protein ingredientsMetabolism disorderOrganic chemistryProlonged action
Owner:EPOQE PHARMA APS
Peptide analogs
ActiveUS11390657B2Prevent unwanted side effectPeptide/protein ingredientsAntibody mimetics/scaffoldsReceptor regulator activityAgonist
Analogs for CLR / RAMP receptor ligands are provided that have agonist, superagonist, antagonist, super-antagonist, or multiple receptor modulating activity. The analogs can be selective for one or more CLR / RAMP receptors, or can be pan-specific for multiple G protein-coupled receptors.
Owner:ADEPTHERA LLC
Polypeptides
ActiveUS20130012427A1Improve solubilityImprove stabilityNervous disorderPeptide/protein ingredientsAmino acidAcid amino sequences
Owner:NOVO NORDISK AS
Compositions and methods for treating acute and chronic pain by local antagonism of CGRP receptors, or combination with sodium channel inhibition or with anti-inflammatory agents
ActiveUS10287337B2Reduce inflammationUndesirable systemic side effectPeptide/protein ingredientsPharmaceutical delivery mechanismSide effectCGRP receptor
Owner:AFASCI
Peptide analogs
Analogs for CLR / RAMP receptor ligands are provided that have agonist, superagonist, antagonist or superantagonist activity. The analogs can be selective for one or more CLR / RAMP receptors, or can be pan-specific.
Owner:ADEPTHERA LLC
Superagonist polypeptide analogs of adrenomedullin and intermedin peptide hormones
Analogs for CLR / RAMP receptor ligands are provided that have agonist, superagonist, antagonist or superantagonist activity. The analogs can be selective for one or more CLR / RAMP receptors, or can be pan-specific.
Owner:ADEPTHERA LLC
Protein signatures for distinguishing between bacterial and viral infections
ActiveUS11131671B2Immunoglobulins against cytokines/lymphokines/interferonsImmunoglobulins against bacteriaProtein FeatureViral infection
Owner:MEMED DIAGNOSTICS
Calcitonin mimetics for treating diseases and disorders
InactiveCN110799527AEasy injectionIncreased sensitivityOrganic active ingredientsPeptide/protein ingredientsFood consumptionIngested food
The present invention relates to humanized calcitonin mimetics and their use in treating diabetes (Type I and / or Type II), excess bodyweight, excessive food consumption, metabolic syndrome, rheumatoidarthritis, non-alcoholic steatohepatitis (NASH), non-alcoholic fatty liver disease, alcoholic fatty liver disease, osteoporosis, or osteoarthritis, poorly regulated blood glucose levels, poorly regulated response to glucose tolerance tests, or poor regulation of food intake.
Owner:KEYBIOSCI
CGRP agonist peptides
ActiveUS9951115B2Peptide/protein ingredientsMetabolism disorderAgonist peptideCalcitonin gene-related peptide
The embodiments provide a calcitonin gene-related peptide (CGRP) agonist peptide or pharmaceutically acceptable salt thereof, including pharmaceutical compositions comprising a CGRP agonist peptide. The embodiments further provide treatment methods, including method of treating metabolic disorders and metabolic disorders selected from metabolic syndrome, diabetes and obesity. The methods involve administering to a subject in need thereof an effective amount of CGRP peptide.
Owner:SOARES CHRISTOPHER J
Use of cgrp receptor antagonists in neuroprotection and neurological disorders
Provided herein are treatment methods, including methods of treating nerve damage, methods of neuroprotection, methods of treating glaucoma and methods of lowering LDL levels. The methods generally involve administering to an individual in need thereof an effective amount of a CGRP receptor antagonist peptide or composition.
Owner:克里斯托弗·J·索尔斯
Intermedin-related polypeptide and its use in the prevention and treatment of sepsis
ActiveCN106749612BReduce leakageReduce edemaAntibacterial agentsPeptide/protein ingredientsVascular endotheliumAdrenomedullin 2
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com