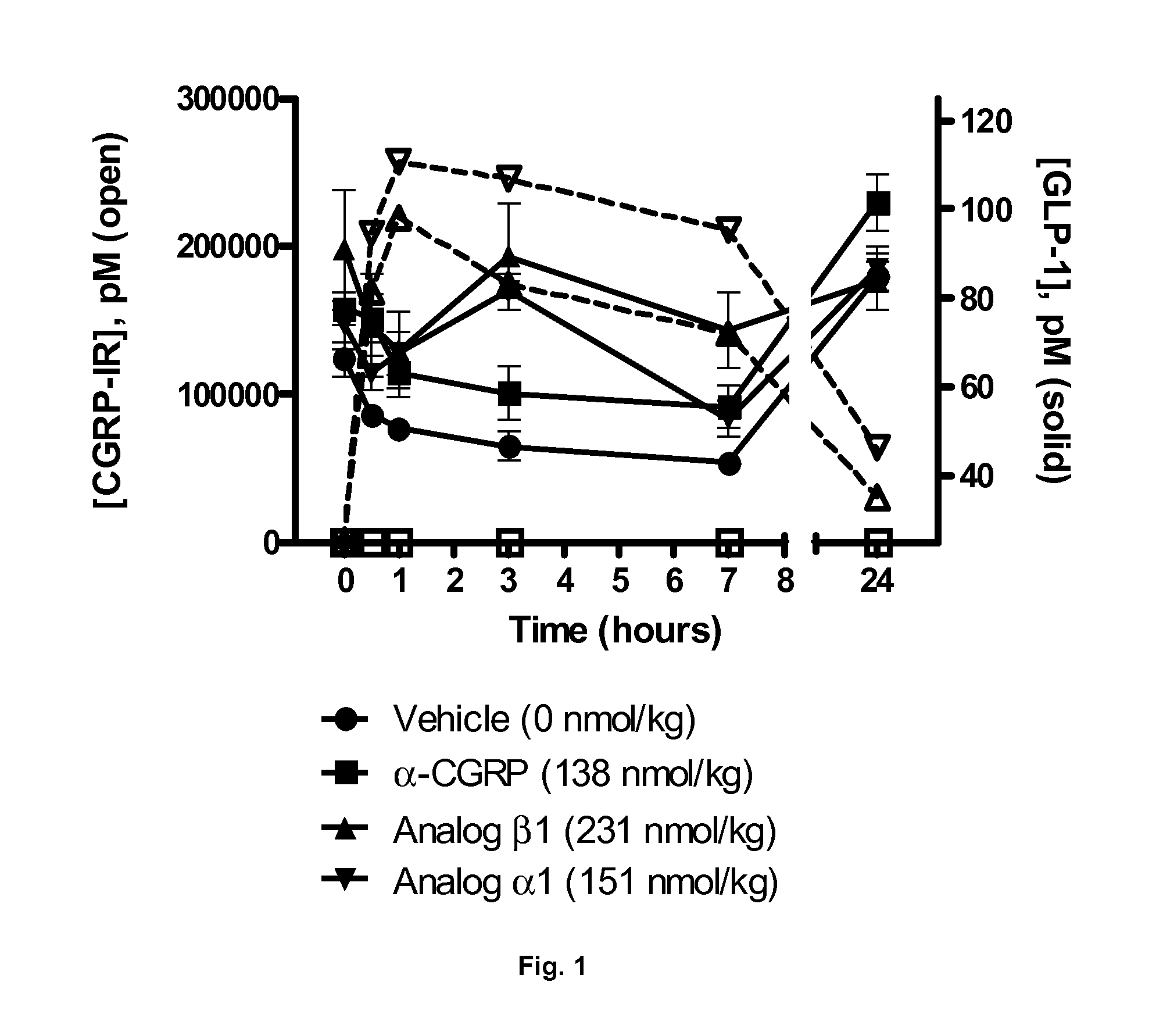
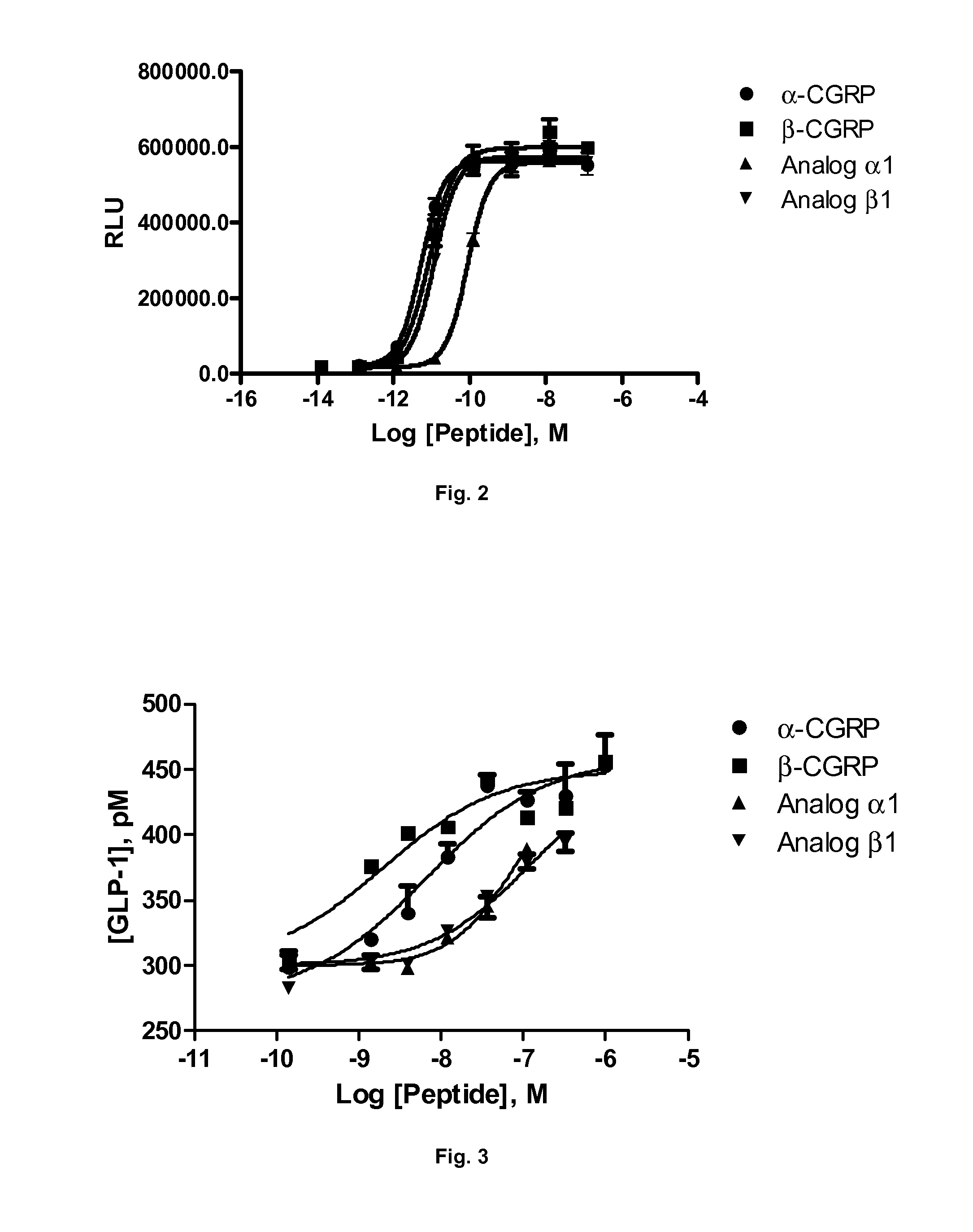
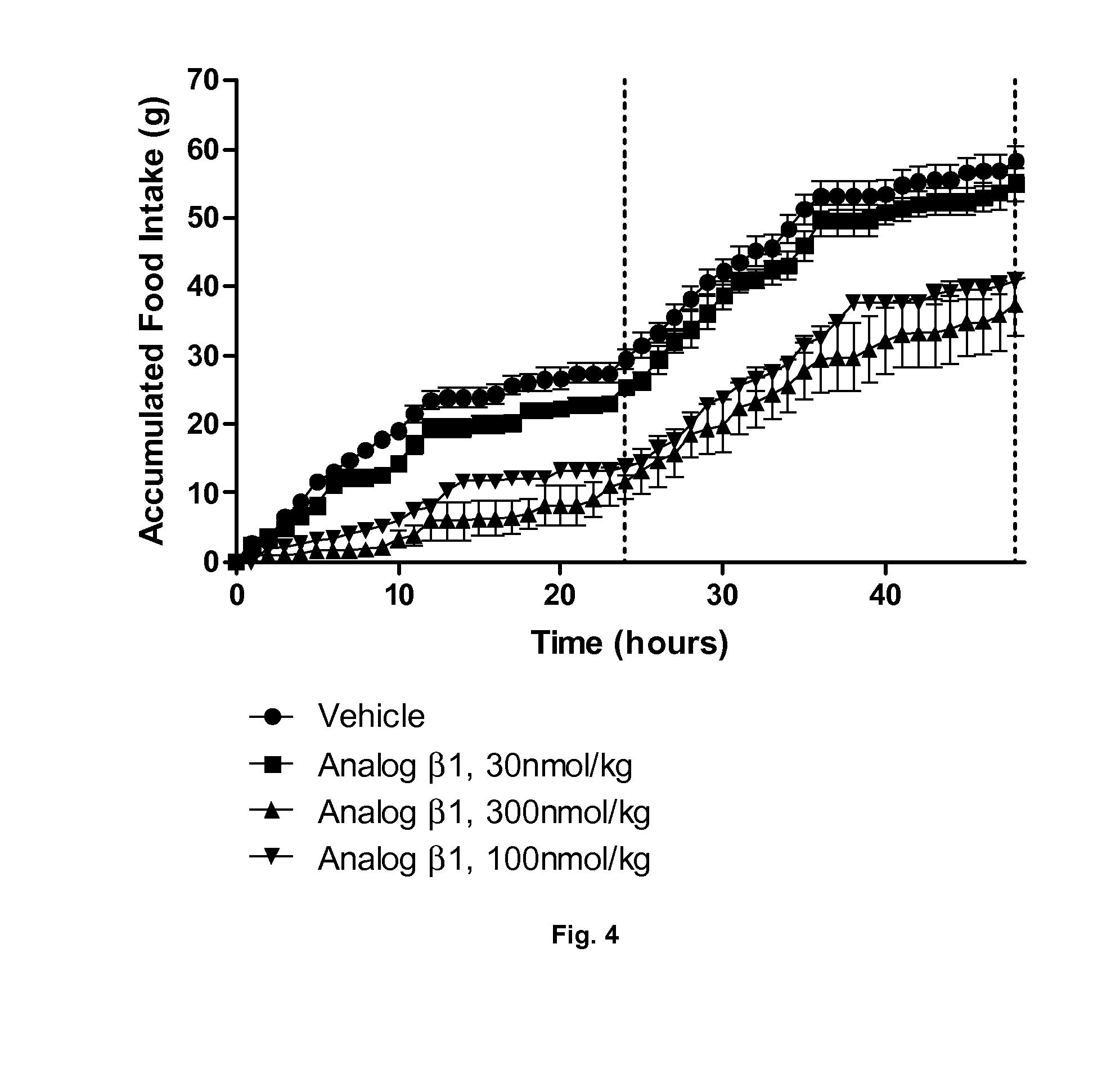
Derivatives of CGRP
a technology of derivatives and cgrp, which is applied in the field of derivatives of cgrp, can solve the problems of preventing the complete understanding of the actions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
N-alpha-[2-(2-{2-[(S)-4-Carboxy-4-(17-carboxyheptadecanoylamino)butyrylamino]ethoxy}ethoxy)acetyl][Ser1]alpha-human CGRP peptide
[0205]
[0206]Preparation: SPPS method A, starting with H-PAL HMPB-ChemMatrix resin (loading 0.43 mmol / g, 0.582 g, 0.25 mmol). The following pseudo prolines employed are available from Novabiochem; Fmoc-Leu-Ser(ψMe,Mepro)-OH and Fmoc-Val-Thr(ψMe,Mepro)-OH. Building block 8-(9-fluorenylmethyloxycarbonylamino)-3,6-dioxaoctanoic acid are commercially available from Iris Biotech and octadecanedioic acid mono tert-butyl ester was prepared as described in WO 2005 / 012347.
[0207]HPLC 08_B2—1: 11.90 min.
[0208]HPLC 08_B4—1: 7.88 min.
[0209]HPLC 04_A4—1: 10.16 min.
[0210]LCMS: m / z=1459.2 (M+3H)3+, 1094.5 (M+4H)4+, 875.6 (M+5H)5+
example 2
N-alpha-[2-(2-{2-[(S)-4-Carboxy-4-(17-carboxyheptadecanoylamino)butyrylamino]ethoxy}ethoxy)acetyl][Ser1]beta-human CGRP peptide
[0211]
[0212]Preparation: SPPS method B starting with ChemMatrix Rink Amide resin (0.25 mmol). The following amino acids were double coupled; glycine (position 20) and glycine (position 14). Building block 8-(9-fluorenylmethyloxycarbonylamino)-3,6-dioxaoctanoic acid are commercially available from Iris Biotech and octadecanedioic acid mono tert-butyl ester was prepared as described in WO 2005 / 012347.
[0213]HPLC 08_B4—1: 8.31 min.
[0214]HPLC 04_A3—1: 13.52 min.
[0215]LCMS: m / z=1461.1 (M+3H)3+, 1096.3 (M+4H)4+
example 3
N-alpha-[2-(2-{2-[(S)-4-Carboxy-4-(17-carboxyheptadecanoylamino)butyrylamino]ethoxy}ethoxy)acetyl]alpha-human CGRP peptide
[0216]
[0217]Preparation: SPPS method A, starting with H-PAL HMPB-ChemMatrix resin (loading 0.43 mmol / g, 0.582 g, 0.25 mmol). The following pseudo prolines employed are available from Novabiochem; Fmoc-Leu-Ser(ψMe,Mepro)-OH and Fmoc-Val-Thr(ψMe,Mepro)-OH. Building block 8-(9-fluorenylmethyloxycarbonylamino)-3,6-dioxaoctanoic acid are commercially available from Iris Biotech and octadecanedioic acid mono tert-butyl ester was prepared as described in WO 2005 / 012347.
[0218]HPLC 08_B4—1: 8.21 min.
[0219]HPLC 08_B2—1: 12.39 min.
[0220]HPLC 05_B5—1: 5.15 min.
[0221]HPLC 04_A3—1: 13.27 min.
[0222]LCMS: m / z=1453.69 (M+3H)3+, 1090.50 (M+4H)4+, 872.42 (M+5)5+
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| energy expenditure | aaaaa | aaaaa |
| resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com