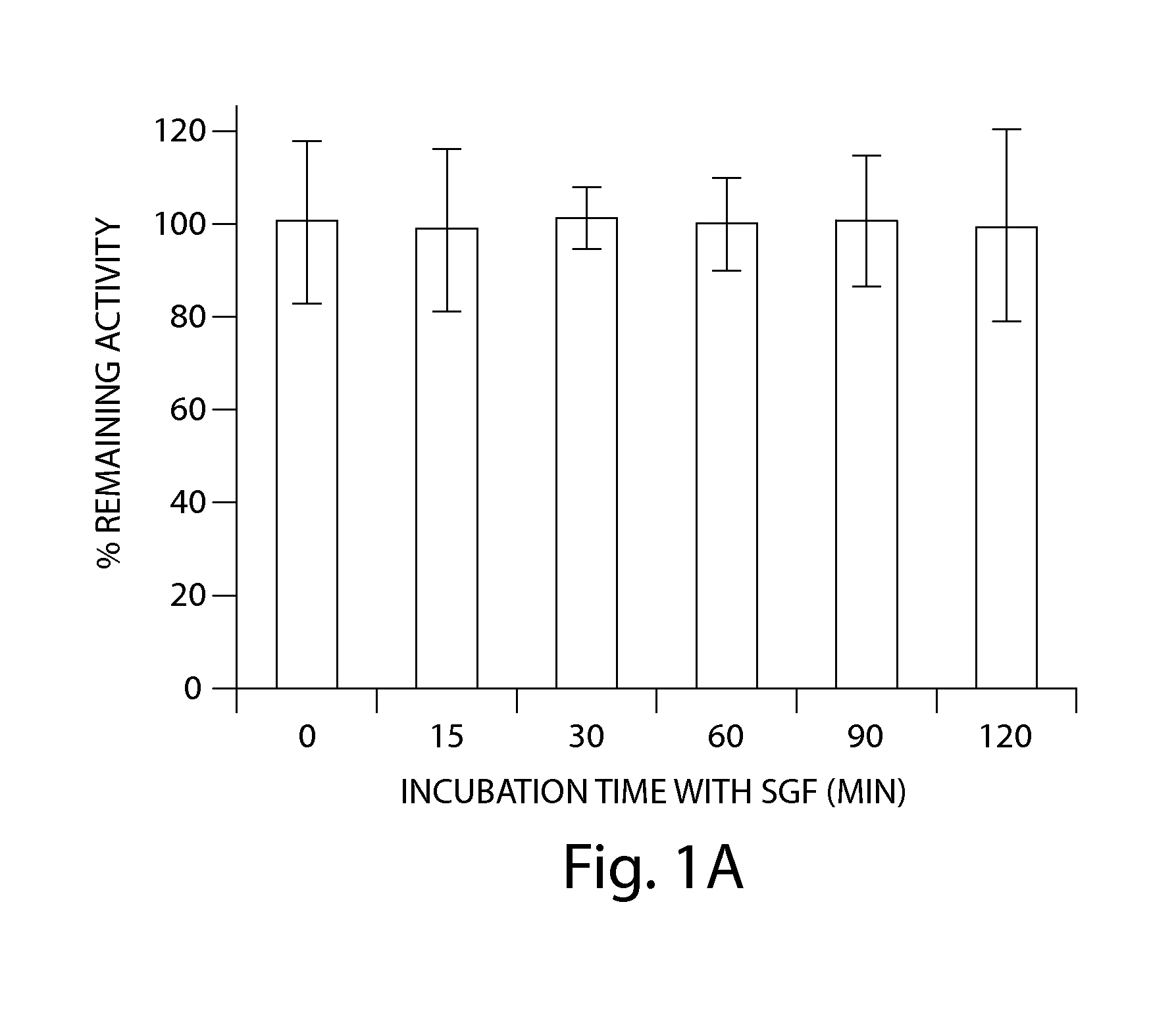
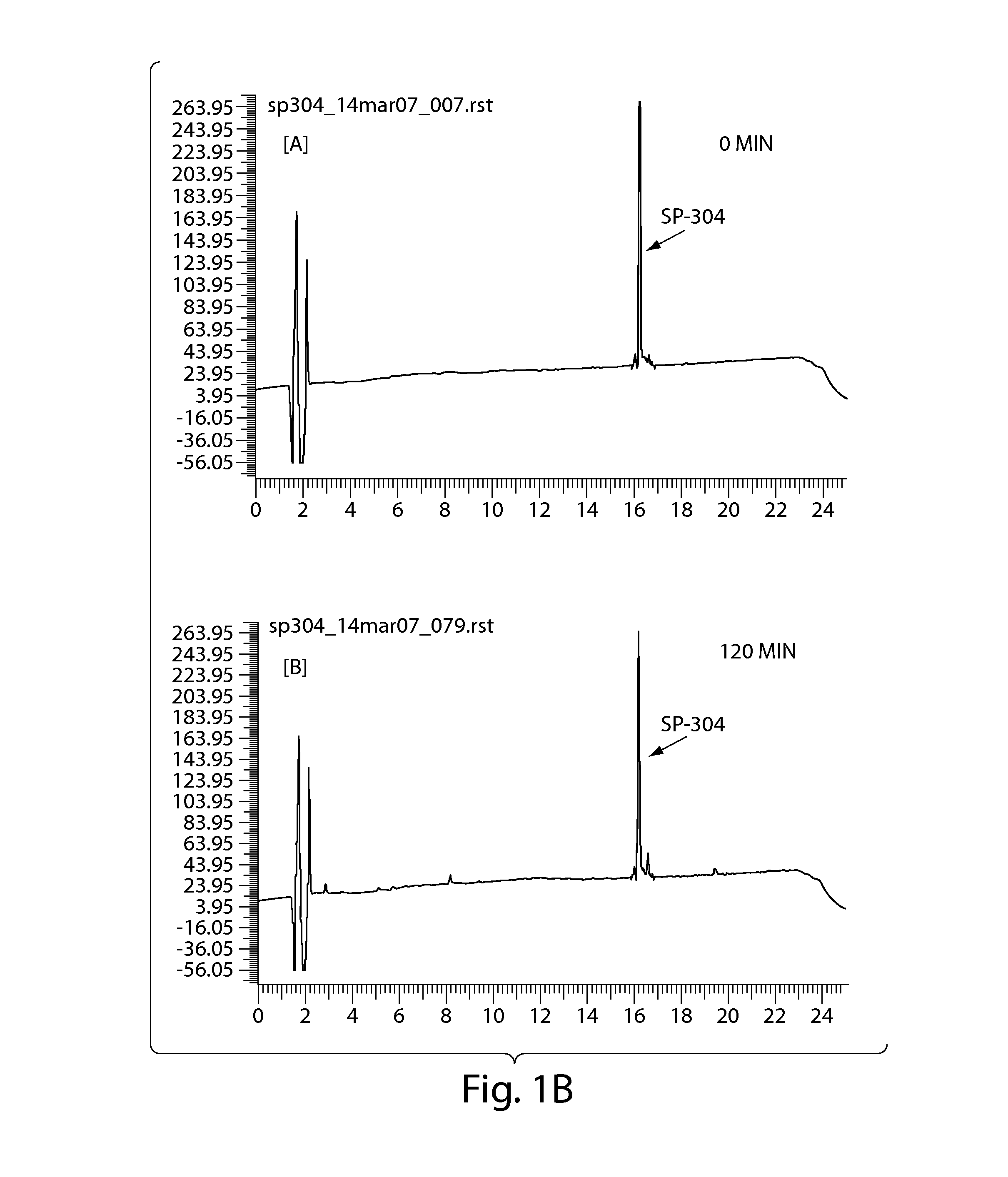
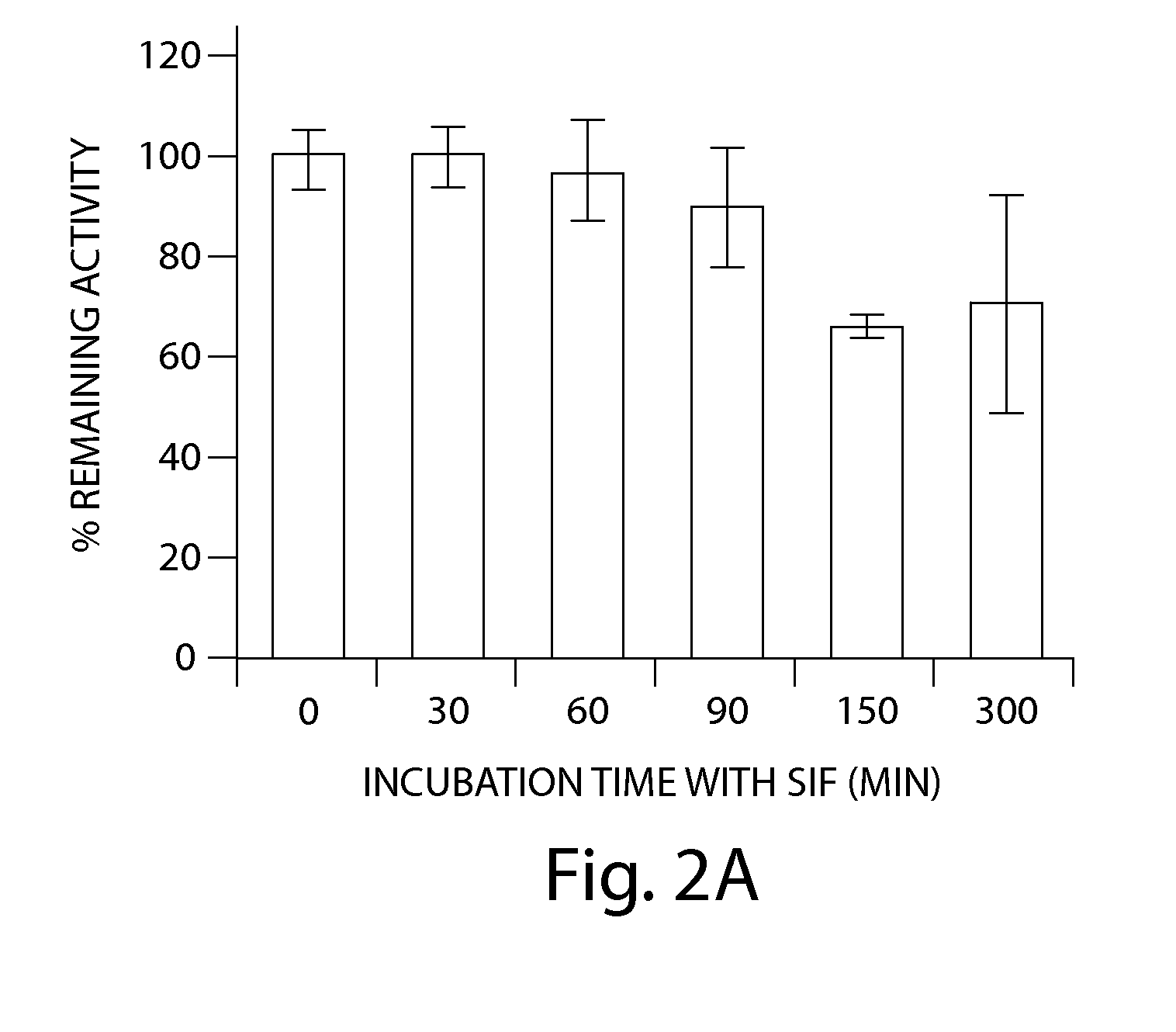
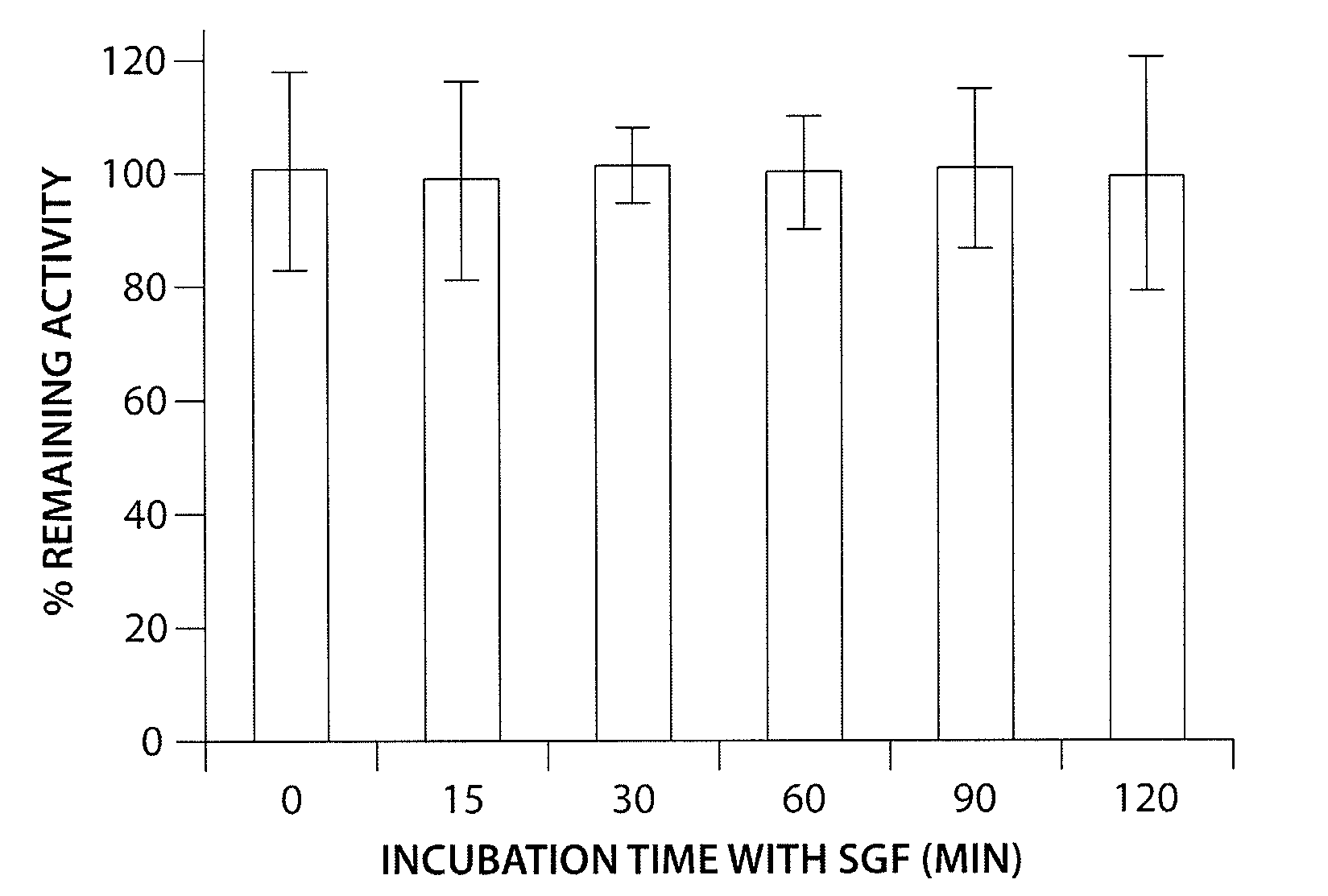
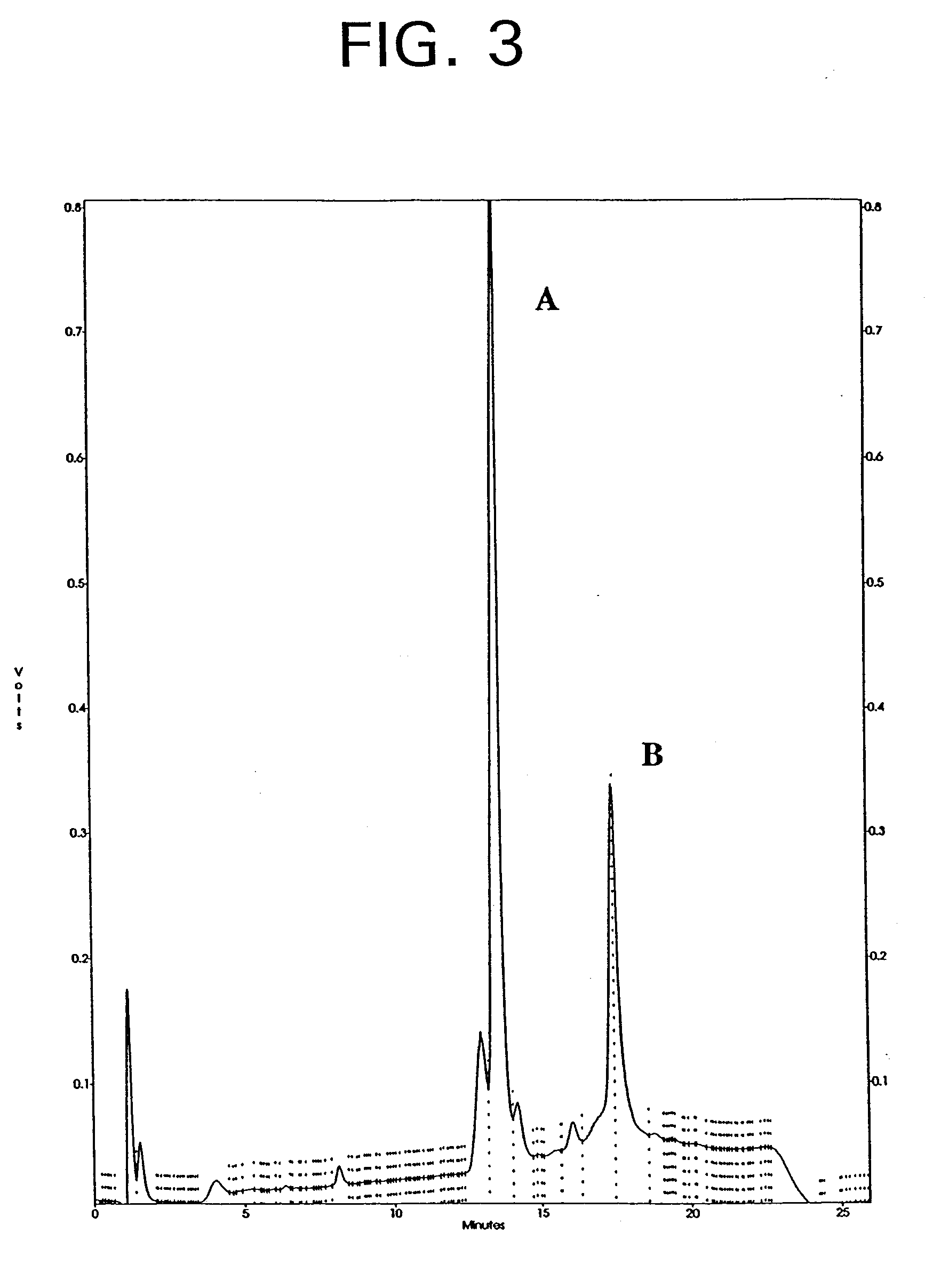
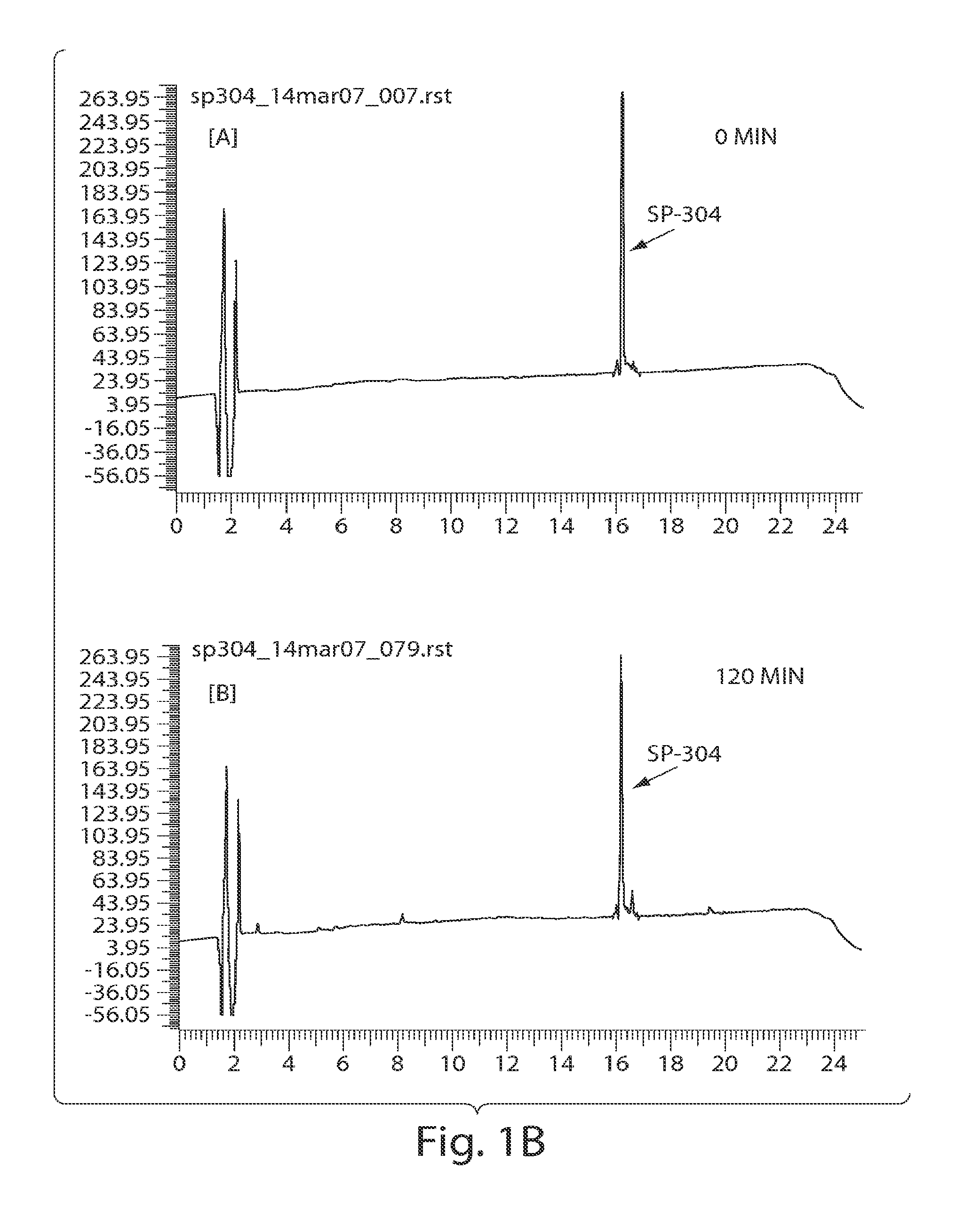
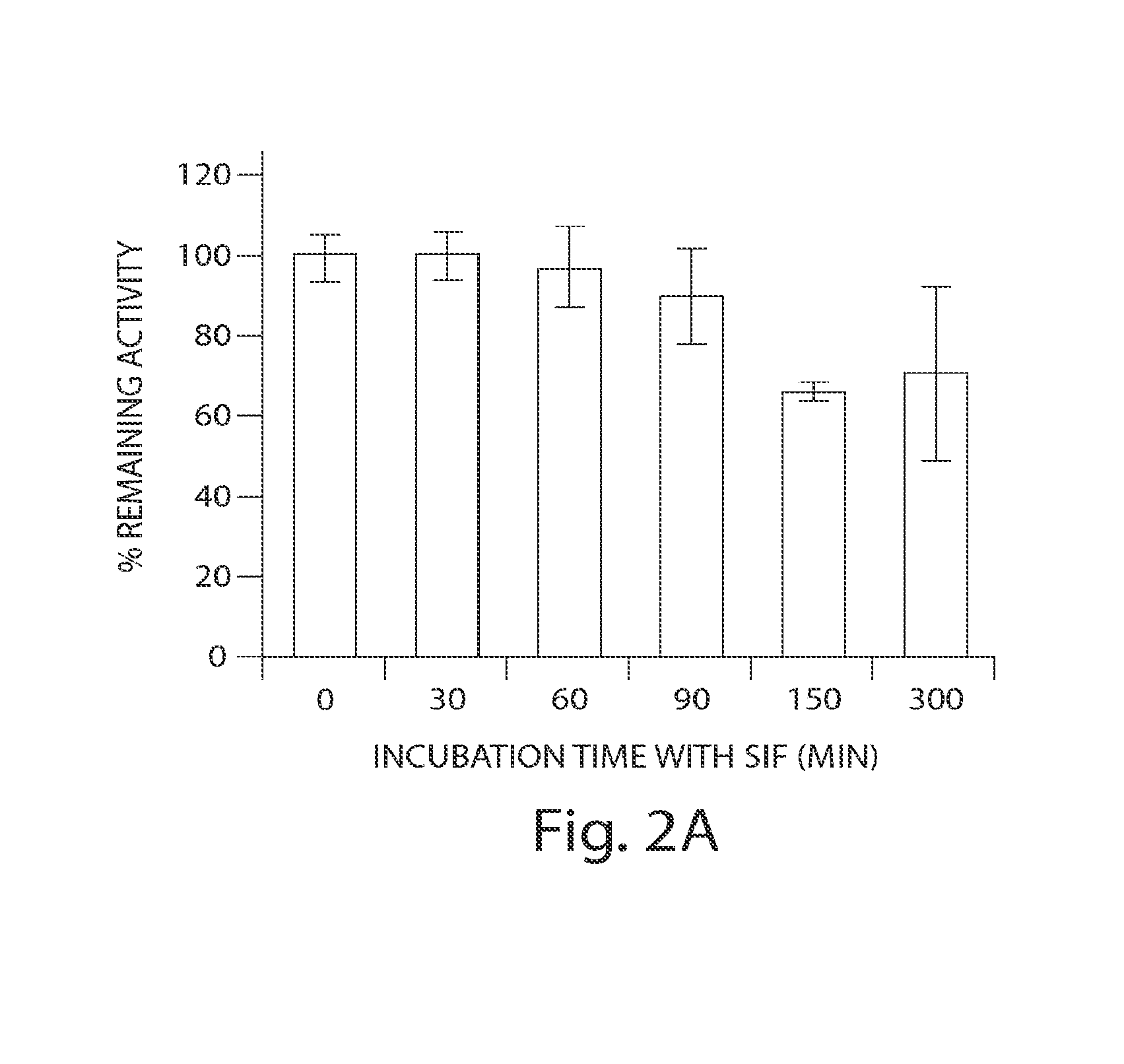
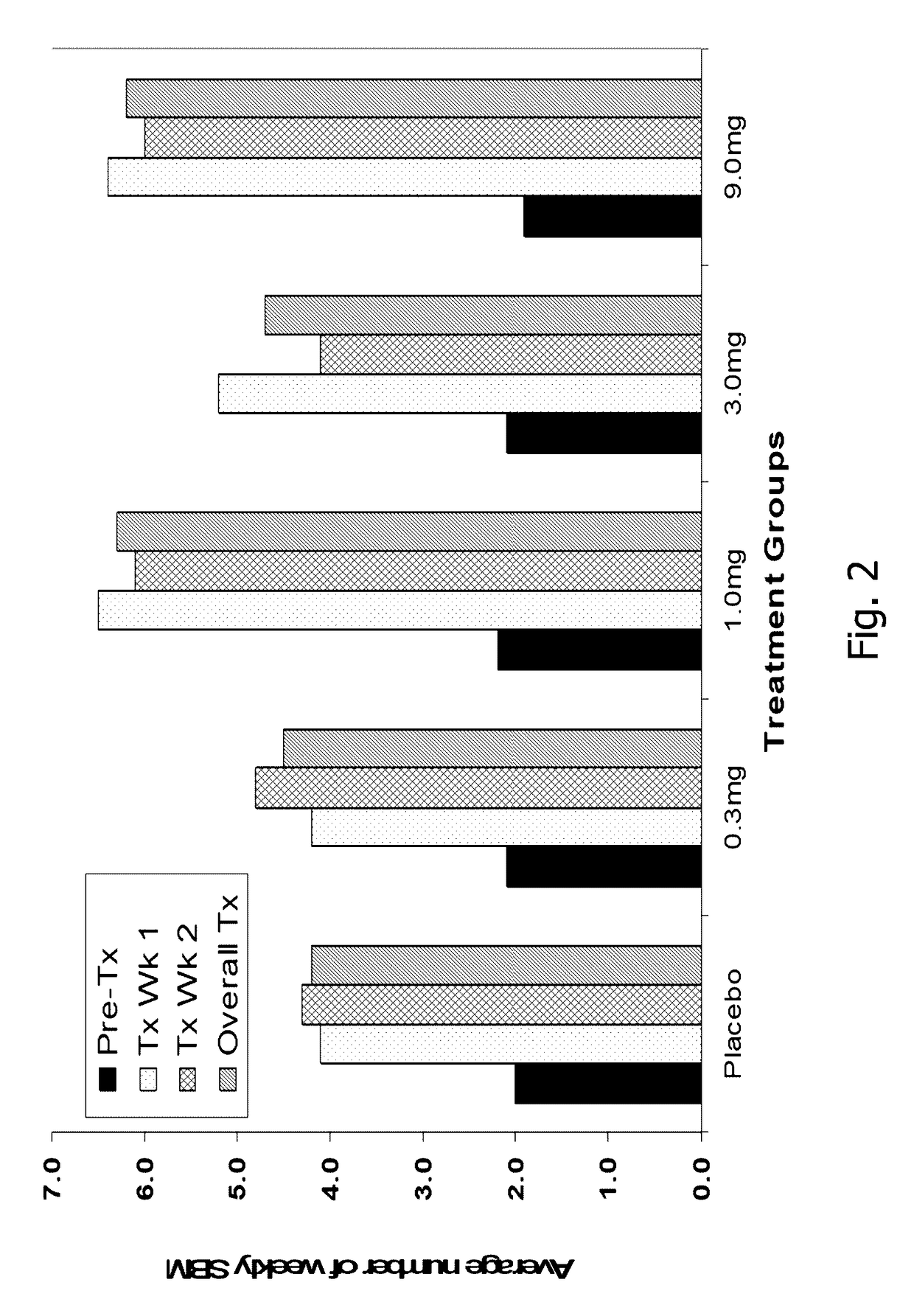
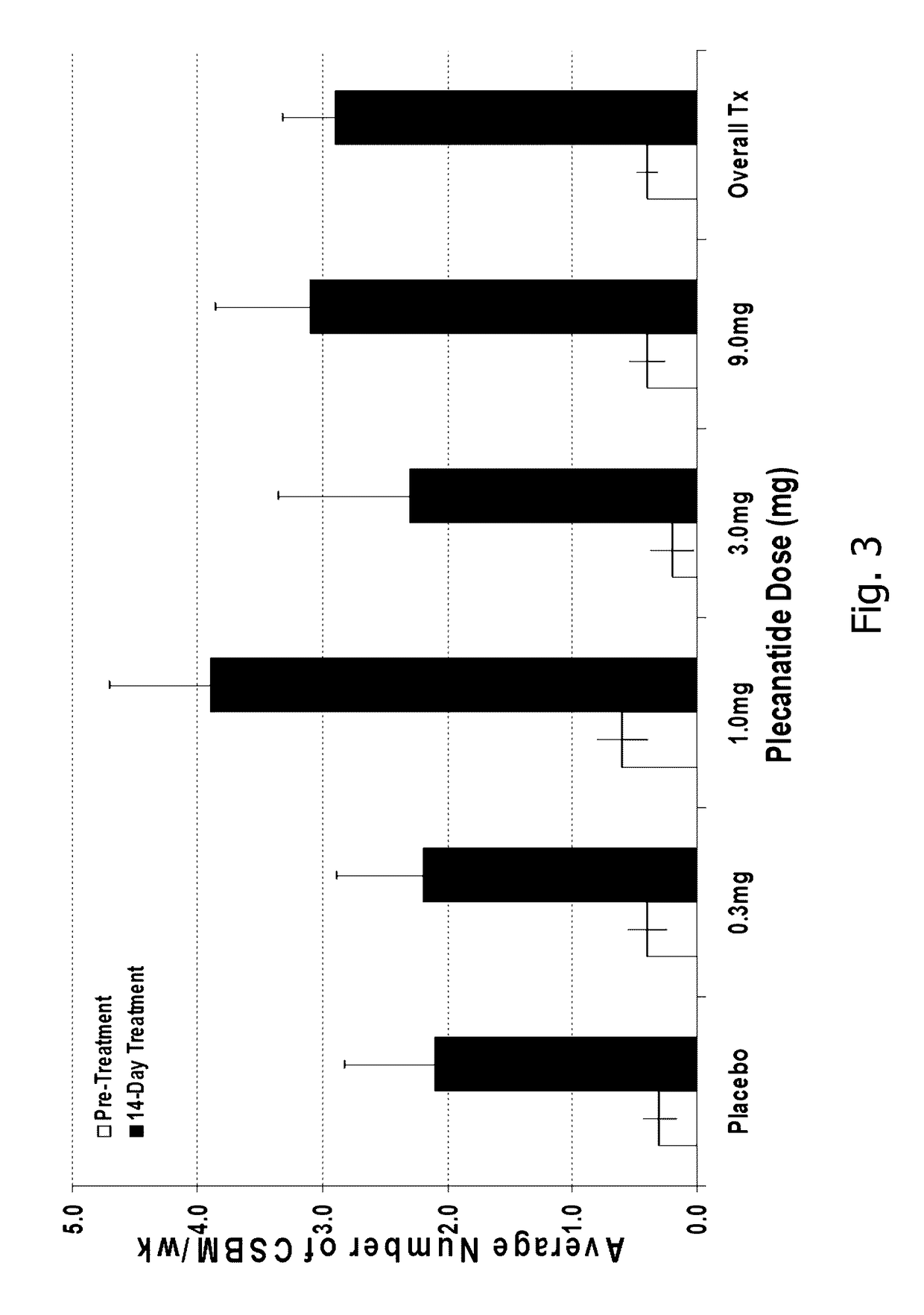
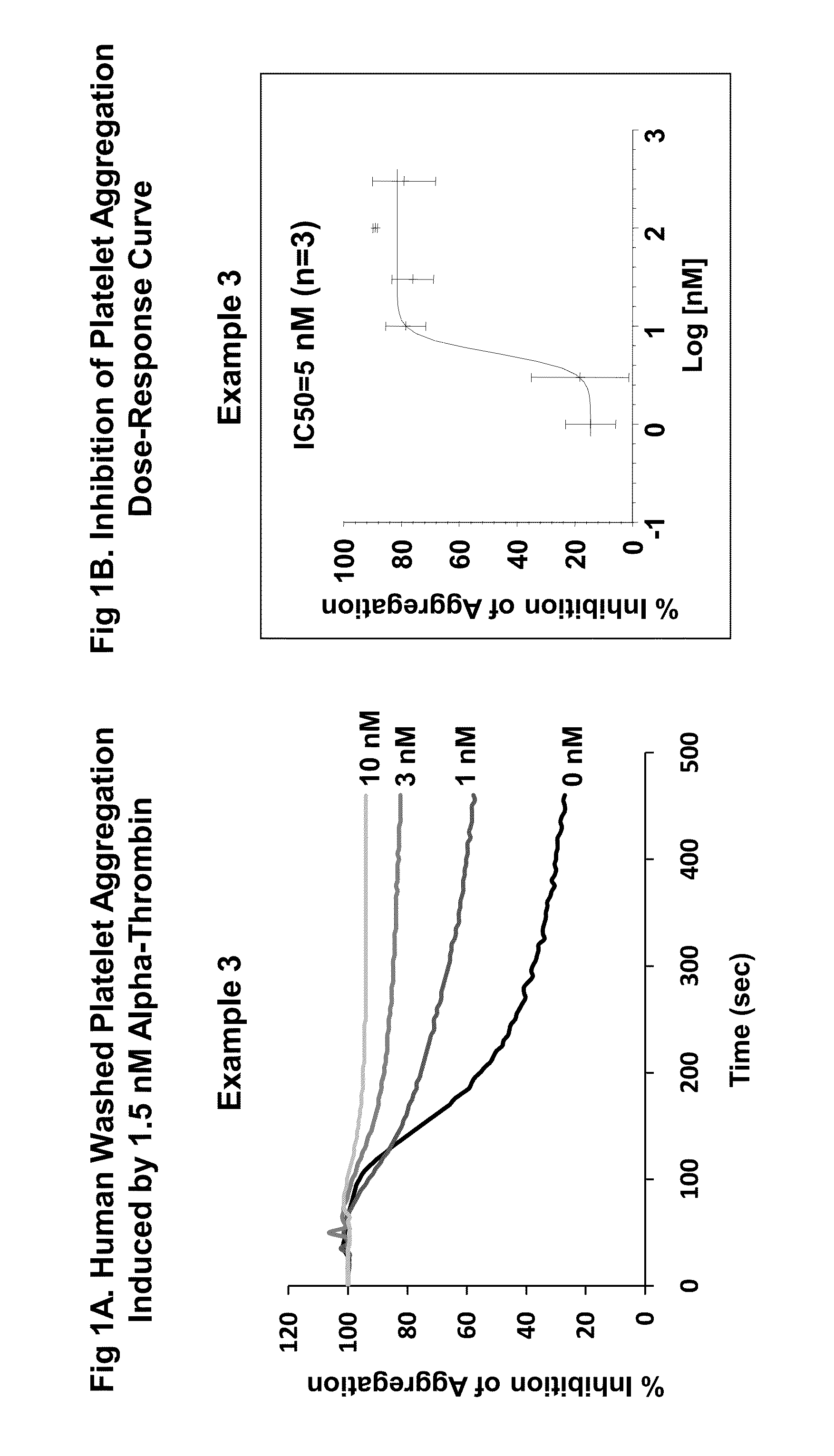
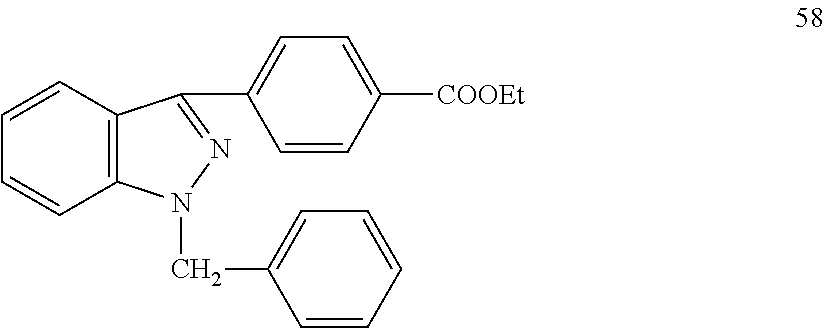
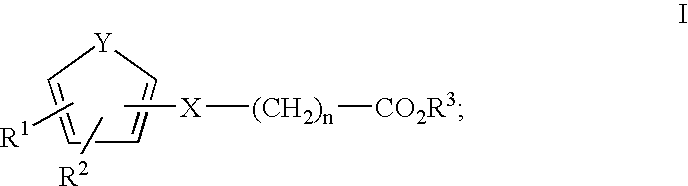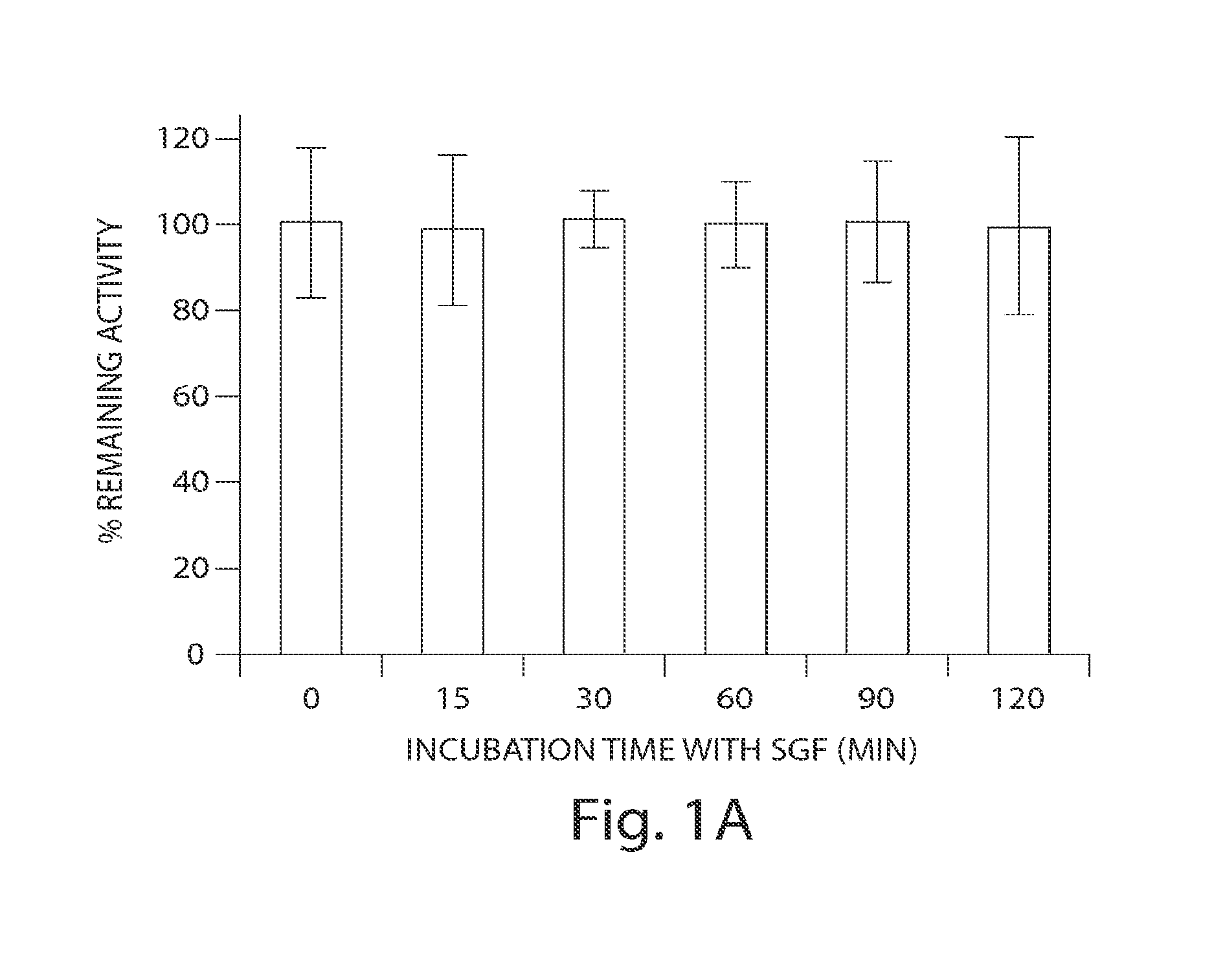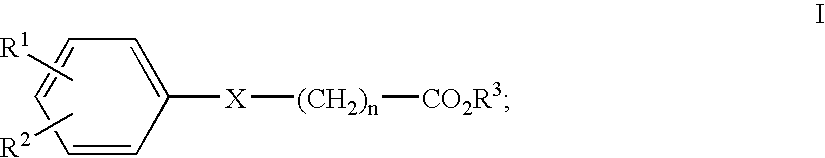Patents
Literature
71 results about "Agonist peptide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Glucagon-like peptide-1 receptor agonists, also known as GLP-1 receptor agonists or incretin mimetics, are agonists of the GLP-1 receptor. This class of medications is used for the treatment of type 2 diabetes.
Formulations of guanylate cyclase c agonists and methods of use
InactiveUS20100221329A1Minimize exposureReducing and eliminating degradationAntibacterial agentsBiocideDiseaseGastrointestinal cancer
The invention provides novel formulations of guanylate cyclase-C (“GCC”) agonist peptides and methods for their use in the treatment of gastrointestinal diseases and disorders, including gastrointestinal cancer. The GCC agonist formulations of the invention can be administered either alone or in combination with one or more additional therapeutic agents, preferably an inhibitor of cGMP-dependent phosphodiesterase or a laxative.
Owner:SYNERGY PHARMA
Agonists of guanylate cyclase useful for the treatment of gastrointestinal disorders, inflammation, cancer and other disorders
ActiveUS20090048175A1Maintain good propertiesIncrease resistanceOrganic active ingredientsSenses disorderPhosphodiesteraseGastrointestinal cancer
The invention provides novel guanylate cyclase-C agonist peptides and their use in the treatment of human diseases including gastrointestinal disorders, inflammation or cancer (e.g., a gastrointestinal cancer). The peptides can be administered either alone or in combination with an inhibitor of cGMP-dependent phosphodiesterase. The gastrointestinal disorder may be classified as either irritable bowel syndrome, constipation, or excessive acidity etc. The gastrointestinal disease may be classified as either inflammatory bowel disease or other GI condition, including Crohn's disease and ulcerative colitis, and cancer.
Owner:BAUSCH HEALTH IRELAND LTD
Formulations of Guanylate Cyclase C Agonists and Methods of Use
ActiveUS20120237593A1Unexpected efficacyMaintain good propertiesAntibacterial agentsSmall article dispensingMedicineAgonist peptide
The invention provides low-dose formulations of guanylate cyclase-C (“GCC”) agonist peptides and methods for their use. The formulations of the invention can be administered either alone or in combination with one or more additional therapeutic agents, preferably an inhibitor of cGMP-dependent phosphodiesterase or a laxative.
Owner:BAUSCH HEALTH IRELAND LTD
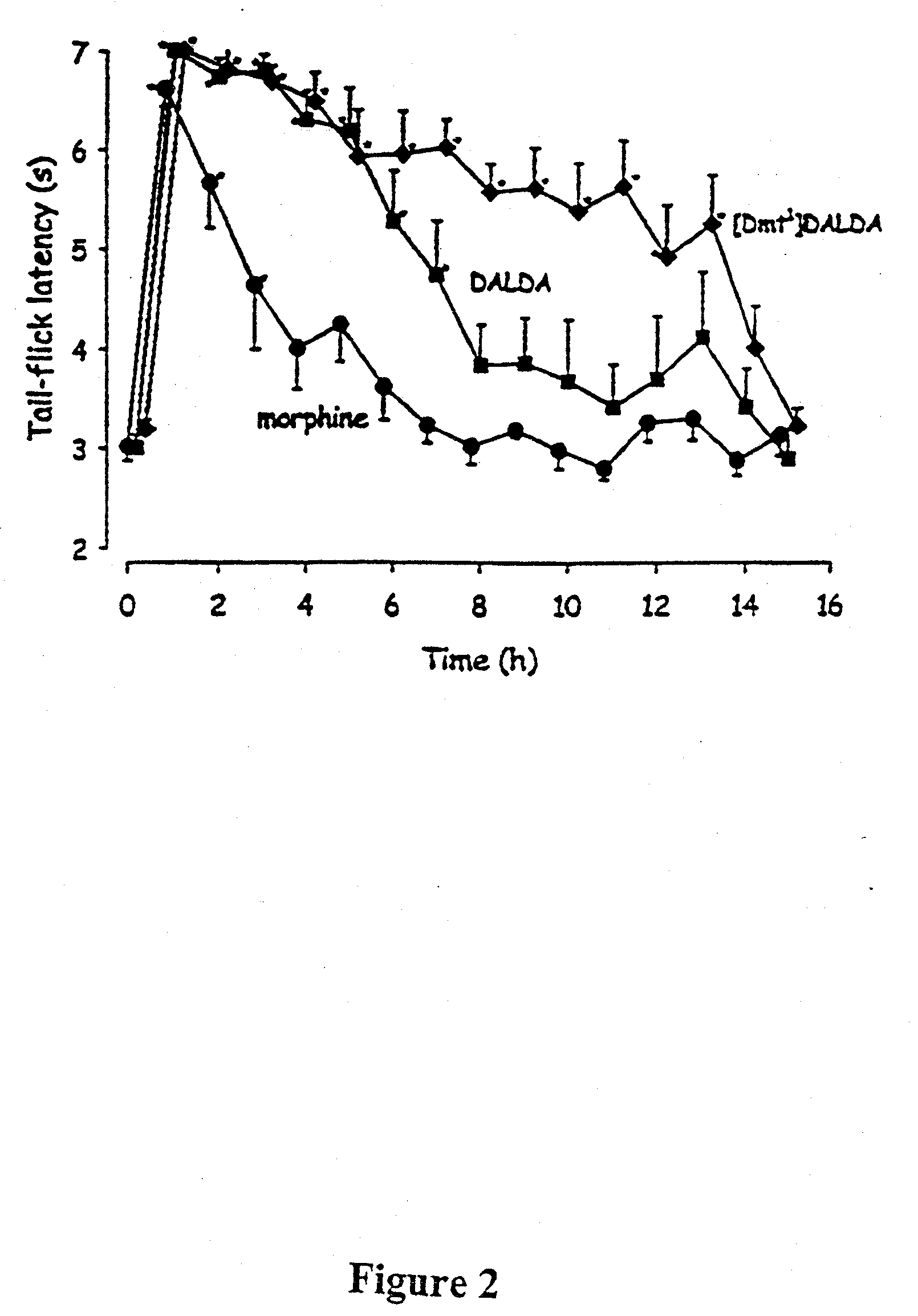
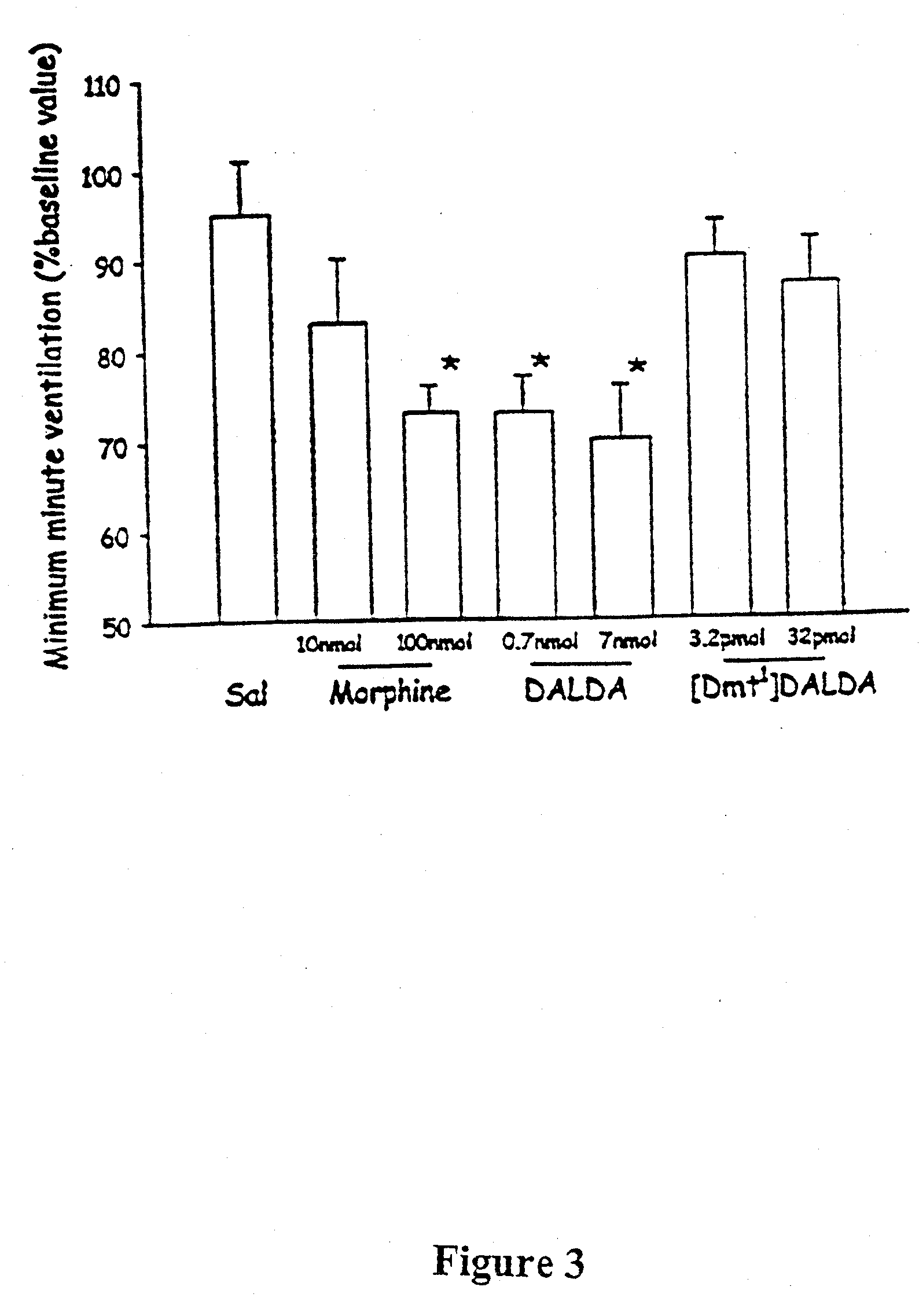
Medicinal uses of mu-opioid receptor agonists
ActiveUS7498297B2Reduce intensityControl rateAntipyreticAnalgesicsDiseaseMu-Opioid Receptor Agonists
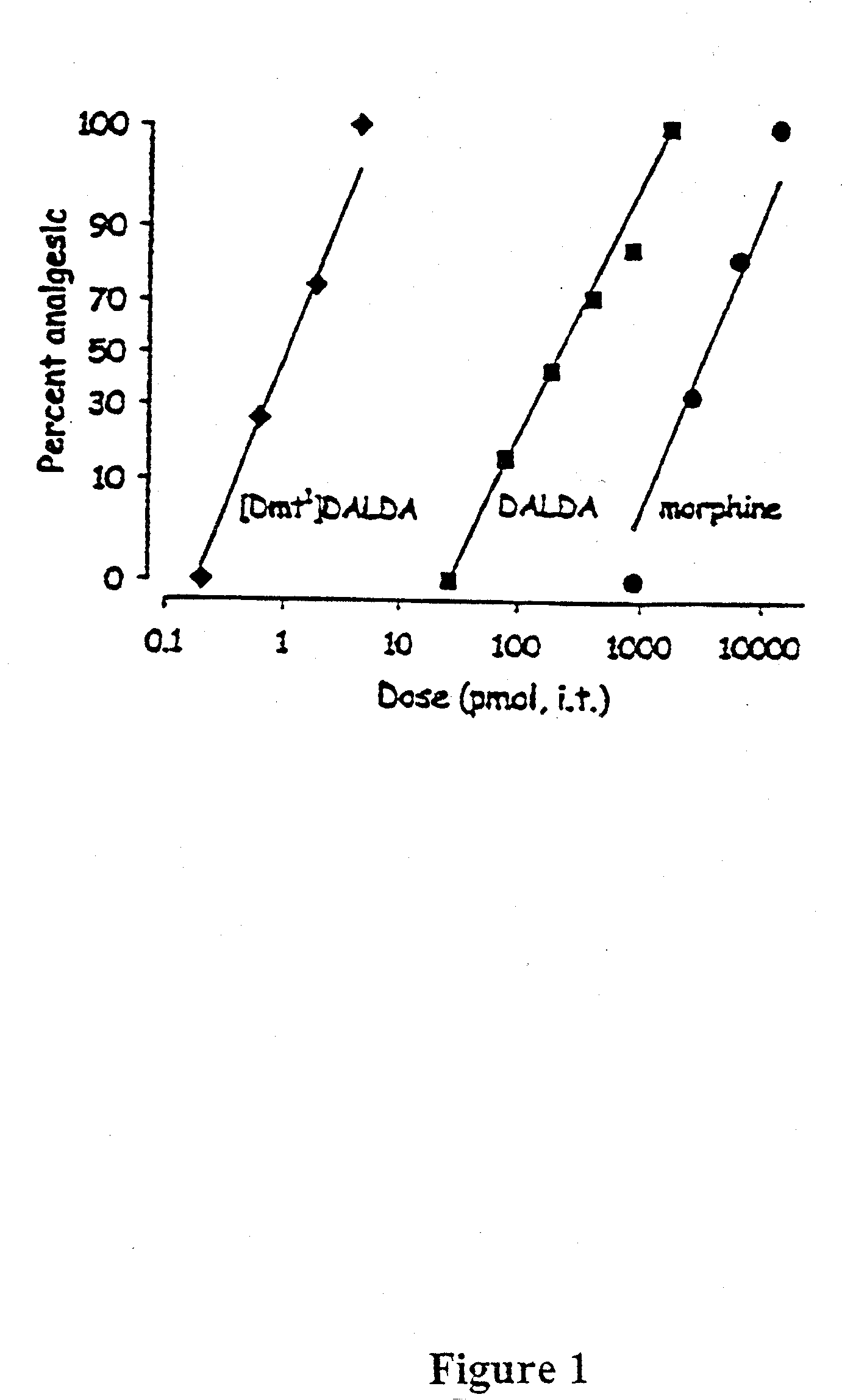
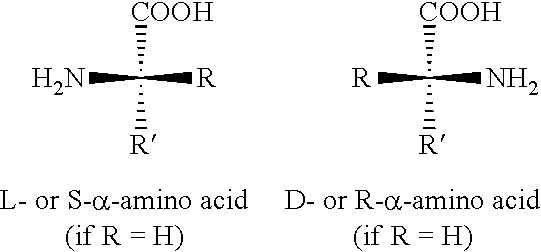
The present invention provides methods for stimulating a mu-opioid receptor agonist peptide in a mammal in need thereof. The methods comprise administering to the mammal an effective amount of a selective mu-opioid receptor agonist peptide that comprises at least two α-amino acid residues. At least one of the amino acid residues has a positive charge. The amino acid residue in the first position is a tyrosine or tyrosine derivative. The amino acid in the second position is a D-α-amino acid. The present invention also provides methods of treating a mammal suffering from conditions or diseases by administering to the mammal an effective amount of the peptides.
Owner:CORNELL RES FOUNDATION INC +1
Compounds, Methods and Formulations for the Oral Delivery of a Glucagon-Like Peptide (Glp)-1 Compound or a Melanocortin-4 Receptor (Mc4) Agonist Peptide
Owner:EMISPHERE TECH INC
Medicinal Uses of Mu-Opioid Receptor Agonists
InactiveUS20070027070A1Reduce intensityControl rateOrganic active ingredientsNervous disorderMu-Opioid Receptor AgonistsMedicine
The present invention provides methods for stimulating mu-opioid receptors with agonist peptides in a mammal in need thereof. The methods comprise administering to the mammal an effective amount of a selective mu-opioid receptor agonist peptide that comprises at least two α-amino acid residues. At least one of the amino acid residues has a positive charge. The amino acid residue in the first position is a tyrosine or tyrosine derivative. The amino acid in the second position is a D-α-amino acid. The present invention also provides methods of treating a mammal suffering from conditions or diseases by administering to the mammal an effective amount of the peptides.
Owner:CORNELL RES FOUNDATION INC +1
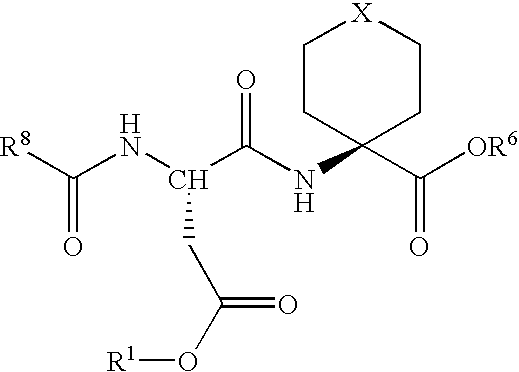
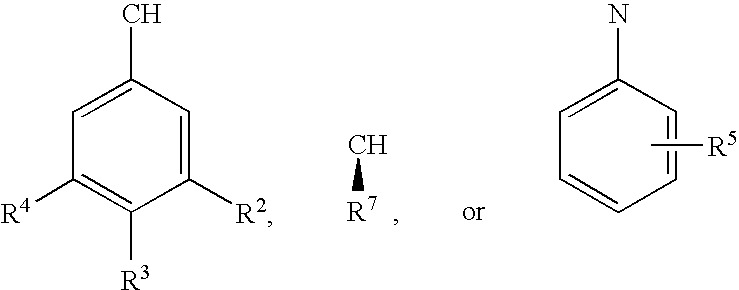
Methods for the synthesis of cyclic peptides
InactiveUS20080287649A1Inhibition formationEffective and efficient for preparationPeptide preparation methodsCyclic peptide ingredientsCyclic peptideDipeptide
Methods for the synthesis of cyclic peptides are provided, as well as novel dipeptide compounds. The methods include the solid phase synthesis of a dipeptide, which is the coupled to a second peptide in a solid phase reaction. The peptide is then cyclized following the coupling reaction. The methods and dipeptides are particularly useful for the synthesis of MC-4 receptor agonist peptides.
Owner:ROCHE PALO ALTO LLC
Agonist peptides of carcinoembryonic antigen (CEA) and nucleic acid sequences encoding the agonist peptides
InactiveUS7723096B2Reduce eliminate CEA-specific T-cellReduce or eliminate CEA-specific T-cell activation and killingBacteriaPeptide/protein ingredientsMHC class ICarcinoembryonic antigen
A nucleic acid molecule encoding an amino acid sequence consisting of an agonist of a MHC class I binding native sequence of CEA having an amino acid substitution and enhanced immunogenicity compared to the native sequence is described. A vector comprising the nucleic acid molecule, host cell comprising the vector and a kit comprising the encoded agonist peptide are also described.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
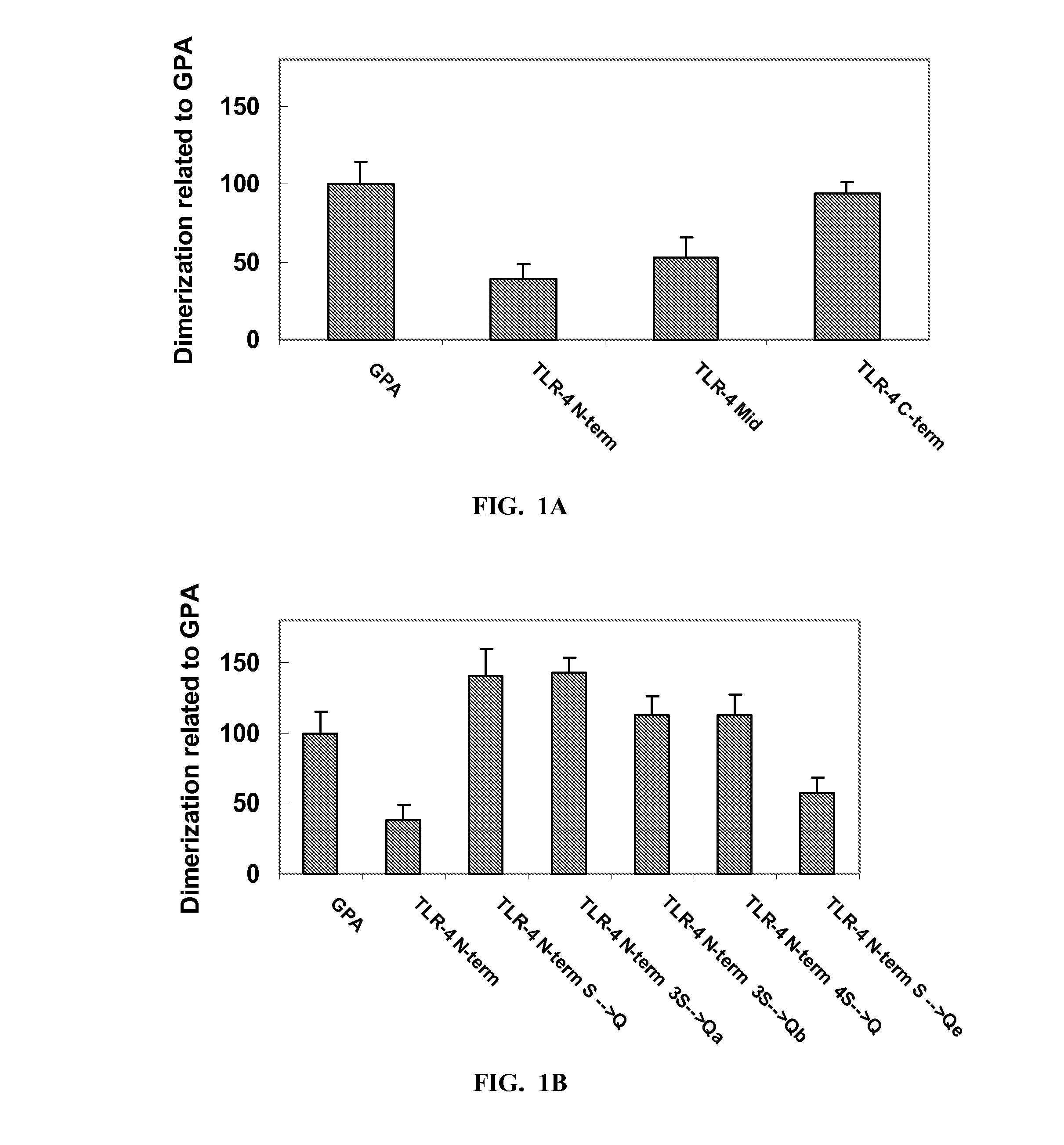
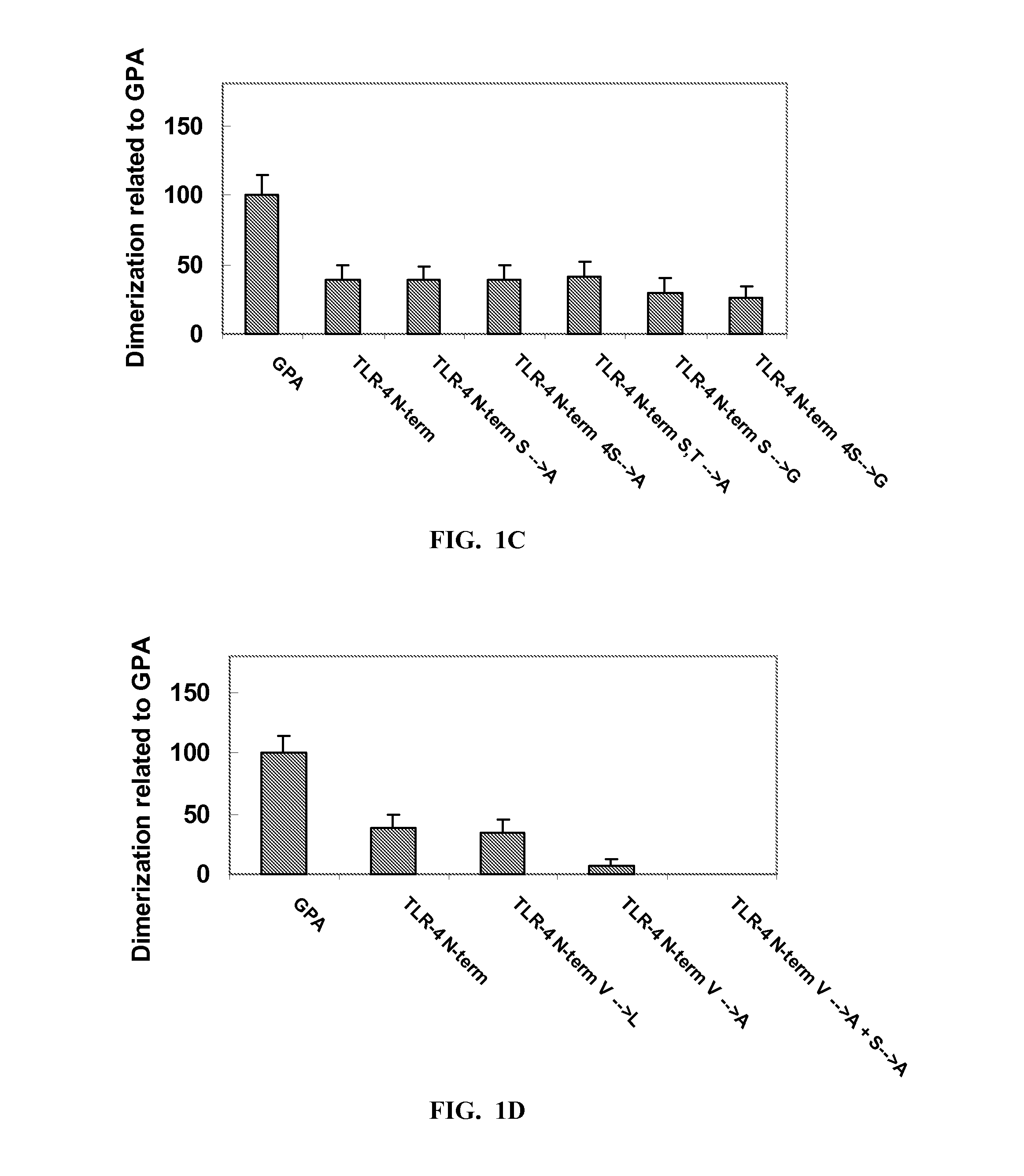
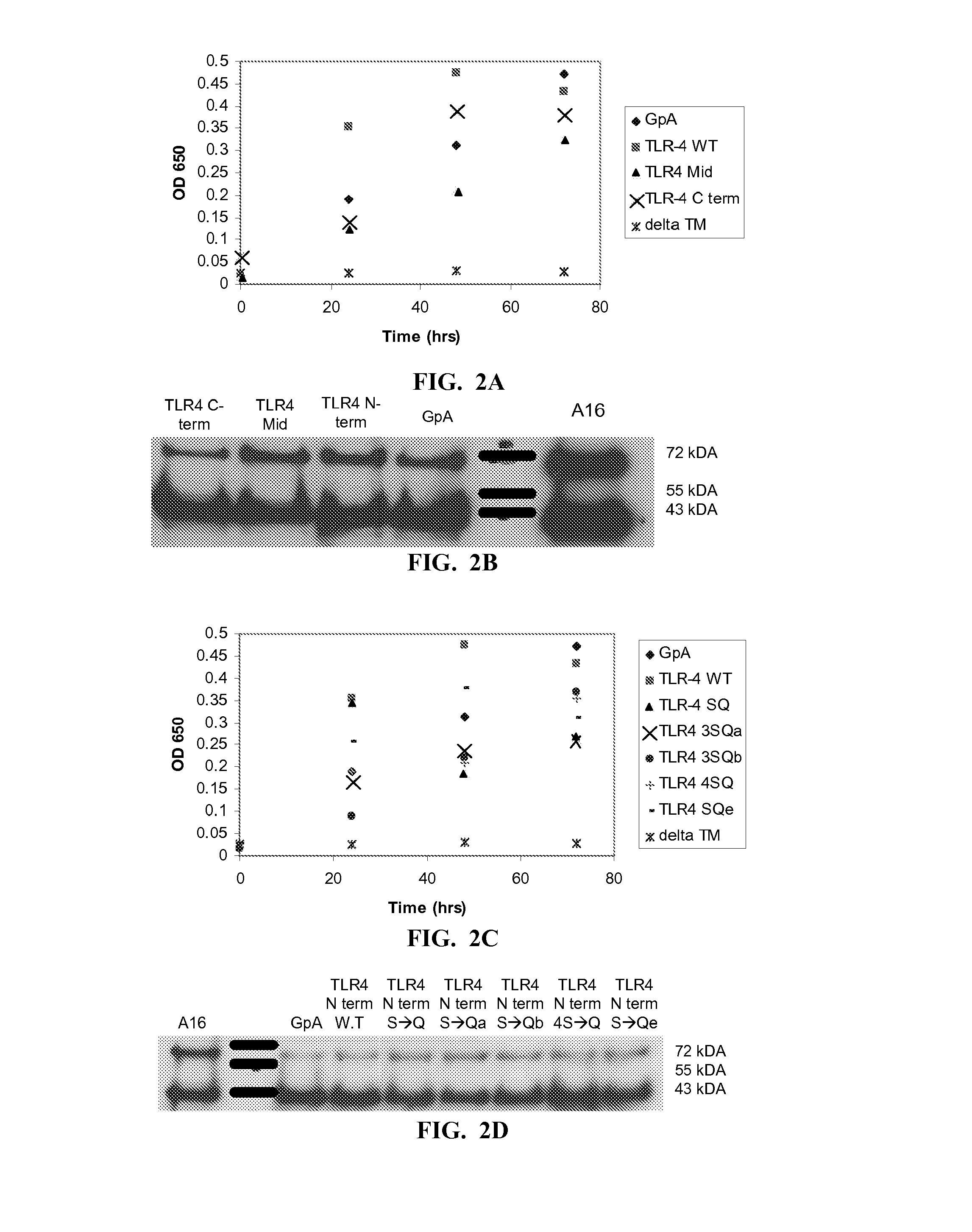
Toll-Like Receptor 4 (Tlr-4) Agonist Peptides For Modulating Tlr-4 Mediated Immune Response
InactiveUS20140220074A1Increase secretionBacterial antigen ingredientsCell receptors/surface-antigens/surface-determinantsInfectious DisorderPharmaceutical drug
Peptides including an amino acid sequence of a fragment of mammalian Toll-like receptor-4 (TLR-4), analogs and derivatives thereof, and pharmaceutical compositions including these peptides. Methods for modulating a TLR-4 mediated immune response, particularly stimulating TLR-4 mediated immune response, thereby treating infectious diseases and cancer.
Owner:YEDA RES & DEV CO LTD
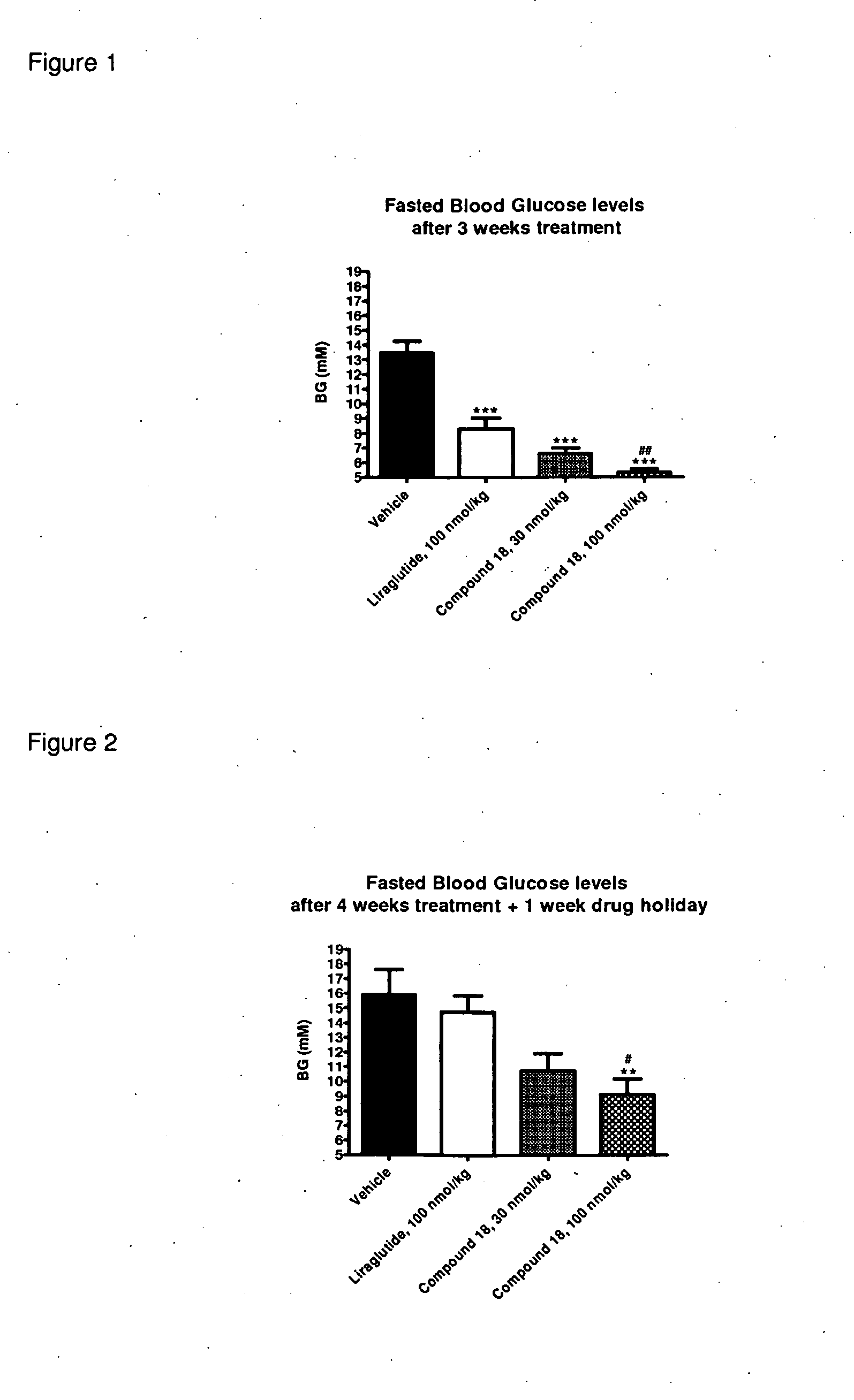
Glp-1 receptor agonist peptide gastrin conjugates
ActiveUS20140336107A1Strong therapeutic activitySenses disorderNervous disorderDiseaseDiabetes mellitus
The present invention relates, inter alia, to certain peptide conjugates, and to the use of the conjugates in the treatment of a variety of diseases or disorders, including diabetes (type 1 and / or type 2) and diabetes-related diseases or disorders.
Owner:ZEALAND PHARM AS
GLP-1 receptor agonist peptide gastrin conjugates
The present invention relates, inter alia, to certain peptide conjugates, and to the use of the conjugates in the treatment of a variety of diseases or disorders, including diabetes (type 1 and / or type 2) and diabetes-related diseases or disorders.
Owner:ZEALAND PHARM AS
Opioid agonist peptides and uses thereof
ActiveUS10278957B2Inhibiting and reducing gastrointestinal motilityOrganic active ingredientsPeptide/protein ingredientsOpioid AgonistAgonist peptide
Owner:PROTAGONIST THERAPEUTICS INC
CXC chemokine receptor 4 agonist peptides
InactiveUS7378098B2Organic active ingredientsPeptide/protein ingredientsHematopoietic cellAgonist peptide
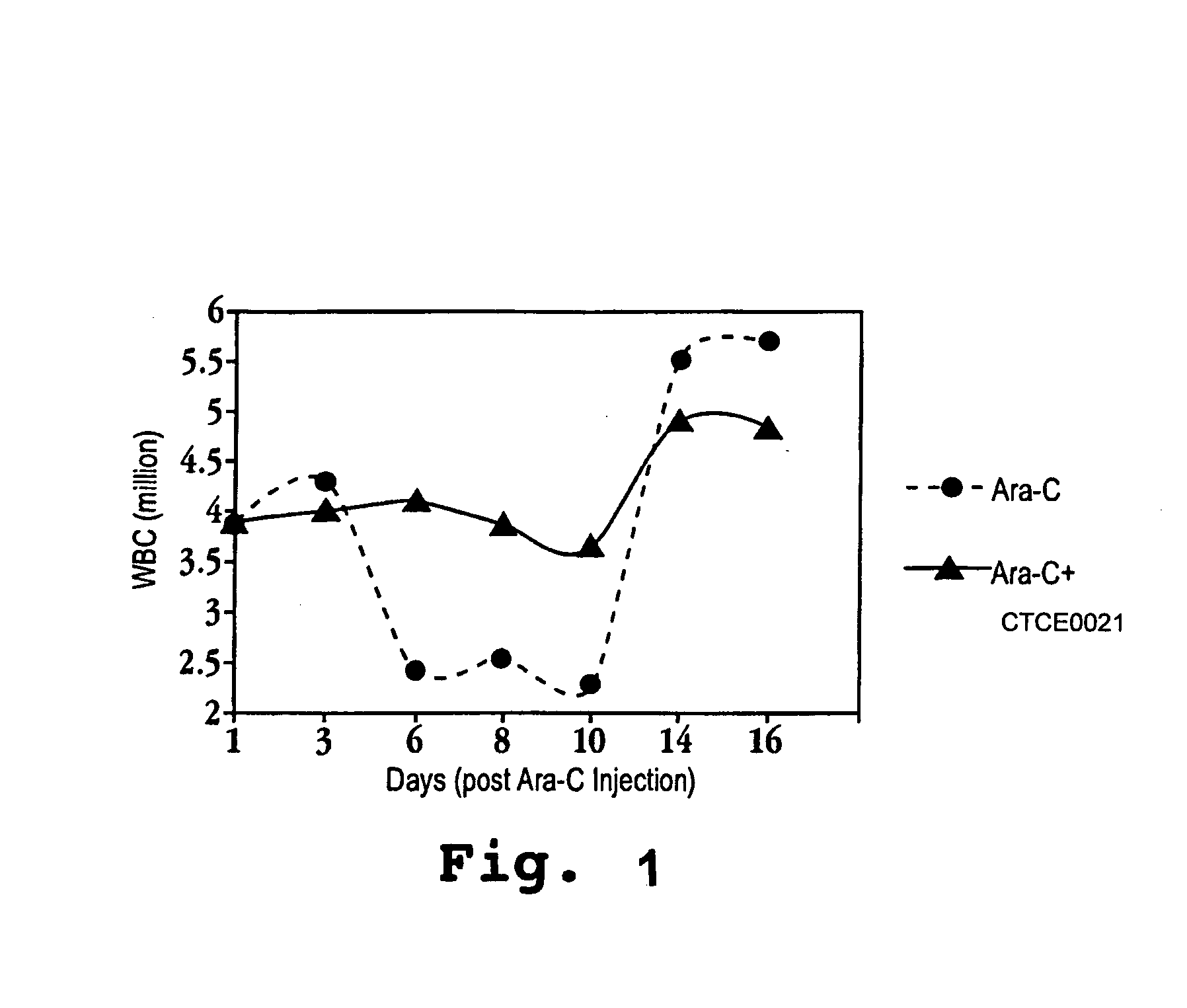
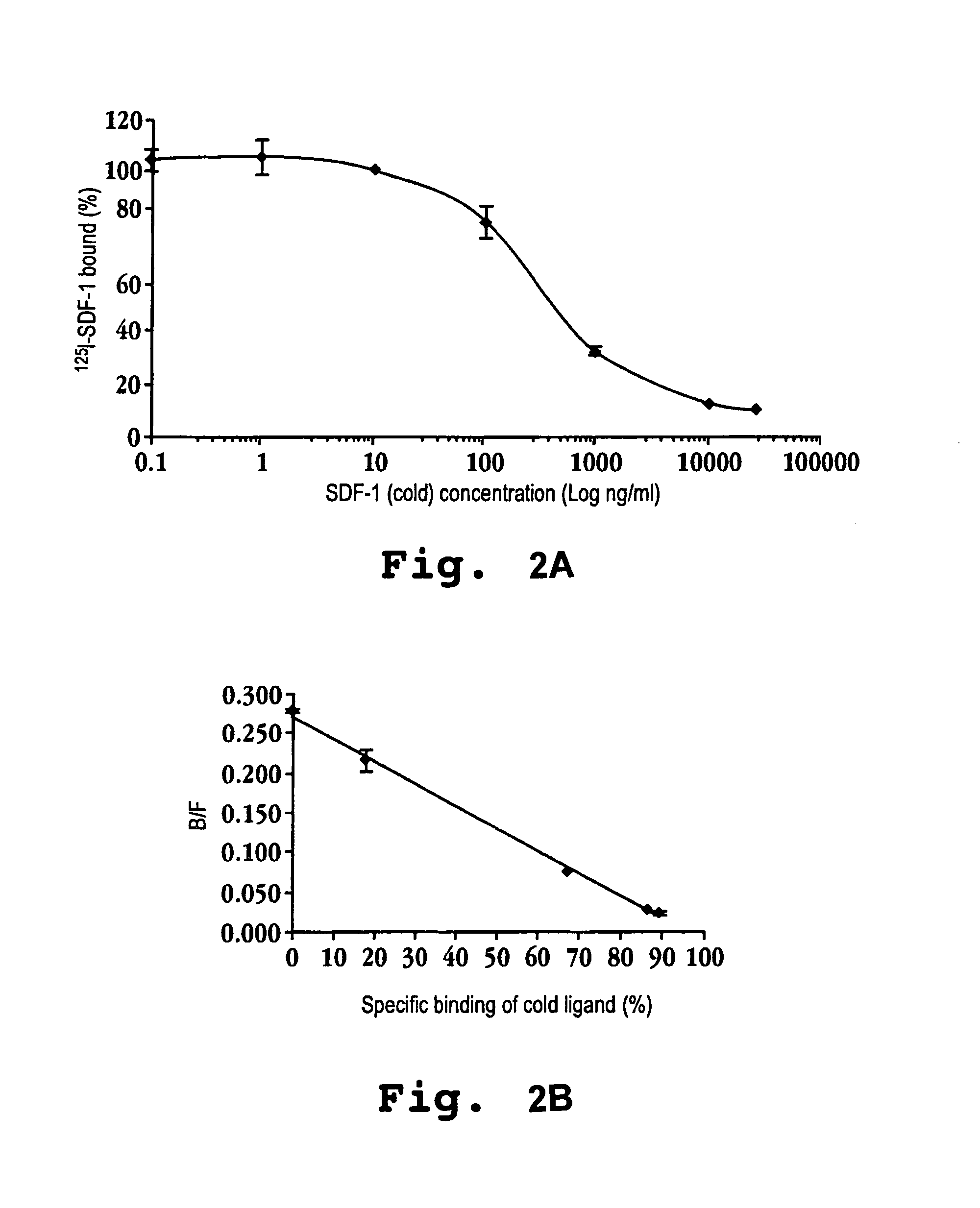
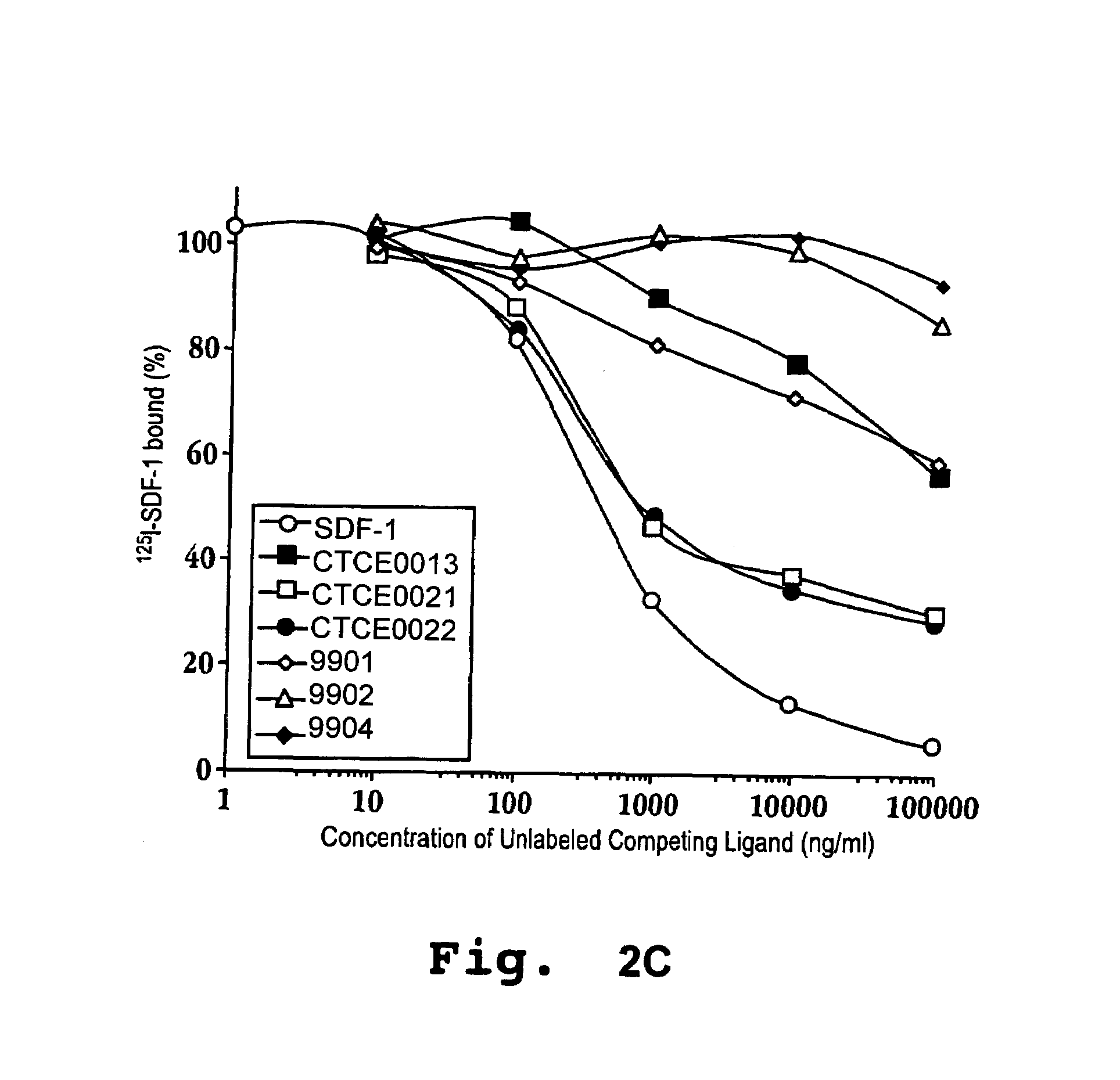
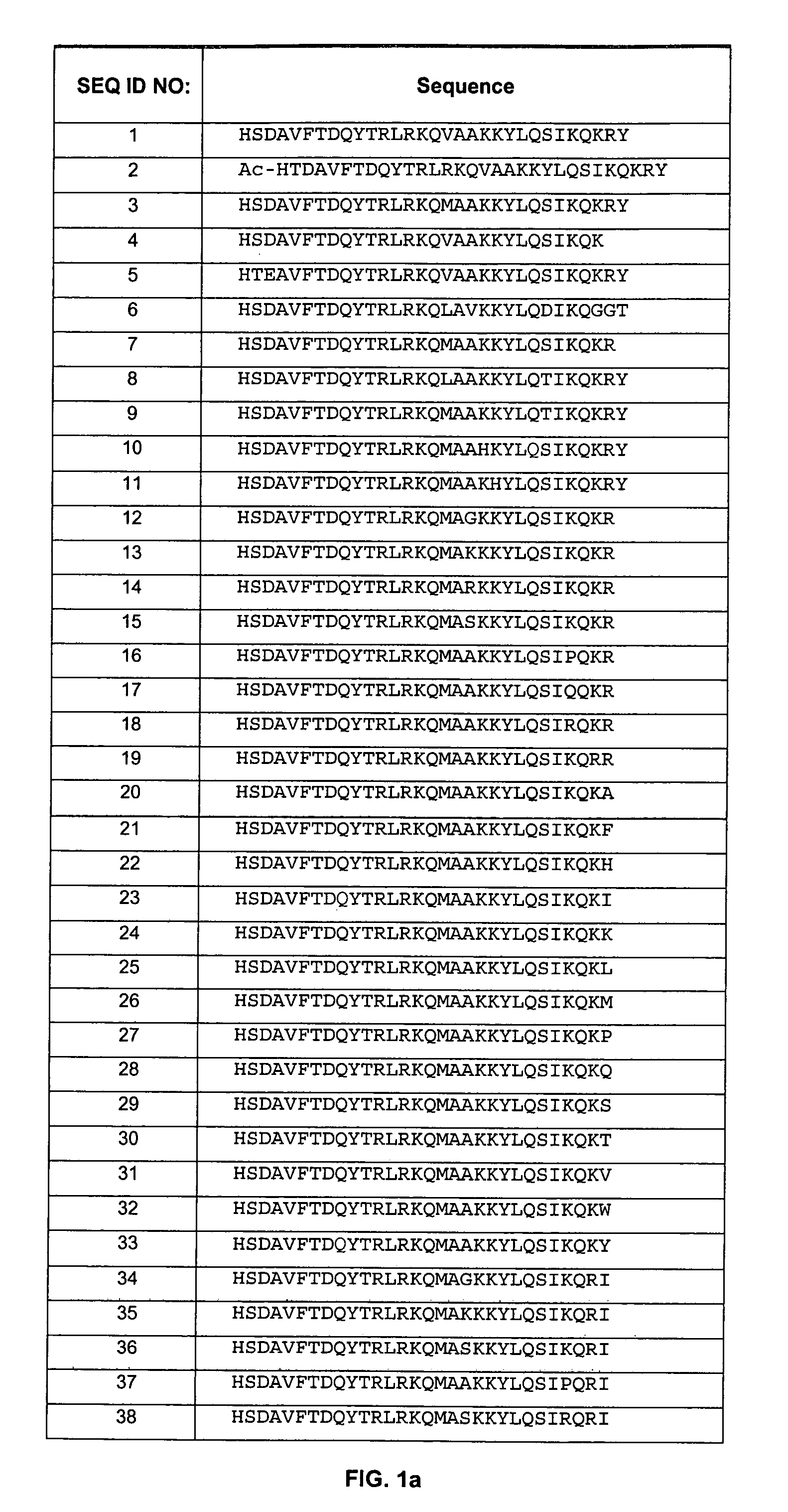
In accordance with various aspects of the invention, CXCR4 agonists, including SDF-1 polypeptides and SDF-1 polypeptide homologues, may be used in reducing the rate of hematopoietic cell multiplication. Methods of the invention may comprise administration of an effective amount of an CXCR4 agonist to cells selected from the group consisting of hematopoietic stem cells and hematopoietic progenitor cells. Cells may be treated in vitro or in vivo in a patient. A therapeutically effective amount of the CXCR4 agonist may be administered to a patient in need of such treatment. Patients in need of such treatments may include, for example patients requiring bone marrow or peripheral blood stem cell transplantation.
Owner:THE UNIV OF BRITISH COLUMBIA +1
Pituitary adenylate cyclase activating peptide (PACAP) receptor (VPAC2) agonist peptide
InactiveUS7378494B2Good potencyFormula stableAntibacterial agentsPeptide/protein ingredientsDiseaseHalf-life
This invention provides novel peptides that function in vivo as agonists of the VPAC2 receptor. These insulin secretagogue polypeptides are shown to lower blood glucose in vivo more than controls upon glucose challenge. The polypeptides of this invention are also stable in formulation and have long half-lives. The peptides of the present invention provide a new therapy for patients with decreased endogenous insulin secretion, in particular type 2 diabetics. In particular, the invention is a polypeptide selected from a specific group of VPAC2-related polypeptides, or functional equivalents thereof. The invention is also directed to a method of treating a metabolic disease in a mammal comprising administering a therapeutically effective amount of the insulin secretagogue peptides to said mammal. Also disclosed are methods of making the peptides, both recombinant and synthetic.
Owner:BAYER HEALTHCARE LLC
Formulations of guanylate cyclase c agonists and methods of use
ActiveUS20150359749A1Effective induction and maintenanceEliminate side effectsAntibacterial agentsPeptide/protein ingredientsDiseaseGastrointestinal cancer
The invention provides novel formulations of guanylate cyclase-C(“GCC”) agonist peptides and methods for their use in the treatment of gastrointestinal diseases and disorders, including gastrointestinal cancer. The GCC agonist formulations of the invention can be administered either alone or in combination with one or more additional therapeutic agents, preferably an inhibitor of cGMP-dependent phosphodiesterase or a laxative.
Owner:BAUSCH HEALTH IRELAND LTD
Compounds, methods and formulations for the oral delivery of a glucagon-like peptide (GLP)-1 compound or a melanocortin-4 receptor (MC4) agonist peptide
Owner:NOVO NORDISK AS
Formulations of guanylate cyclase c agonists and methods of use
ActiveUS20150366935A1Unexpected efficacyMaintain good propertiesAntibacterial agentsPeptidesMedicineAgonist peptide
The invention provides low-dose formulations of guanylate cyclase-C (“GCC”) agonist peptides and methods for their use. The formulations of the invention can be administered either alone or in combination with one or more additional therapeutic agents, preferably an inhibitor of cGMP-dependent phosphodiesterase or a laxative.
Owner:BAUSCH HEALTH IRELAND LTD
Formulations of guanylate cyclase C agonists and methods of use
The invention provides low-dose formulations of guanylate cyclase-C (“GCC”) agonist peptides and methods for their use. The formulations of the invention can be administered either alone or in combination with one or more additional therapeutic agents, preferably an inhibitor of cGMP-dependent phosphodiesterase or a laxative.
Owner:BAUSCH HEALTH IRELAND LTD
Y4 selective receptor agonists for therapeutic interventions
Y4 receptor agonist peptide selected from the group consisting of: [Ala30]PP2-36, [Thr30]PP2-36, [Asn30]PP2-36, [Gln30]PP2-36, [Glu10]PP2-36, [Glu10,Leu17,Thr30]PP2-36, [Nle17,Nle30]PP2-36, [Glu10,Nle17,Nle30]PP2-36, their PP1-36 equivalents, and analogues and derivatives thereof as described in the specification, are selective agonists of the Y4 receptor relative to the Y1 and Y2 receptors, and are useful in the treatment, for example, of obesity and overweight, and conditions in which these are considered contributory factors, and in the treatment of diarrhoea and intestinal hypersecretion.
Owner:7TM PHARM AS
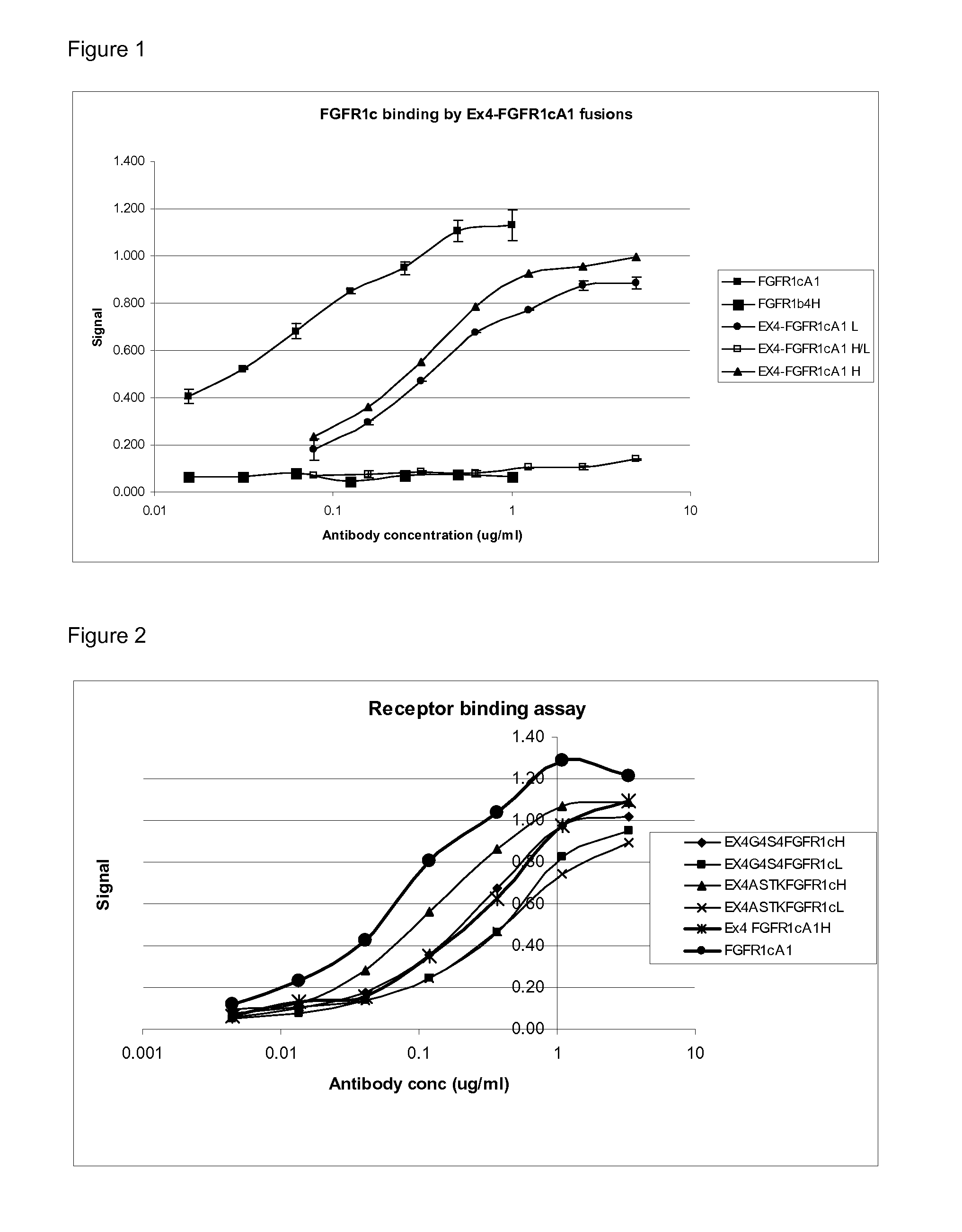
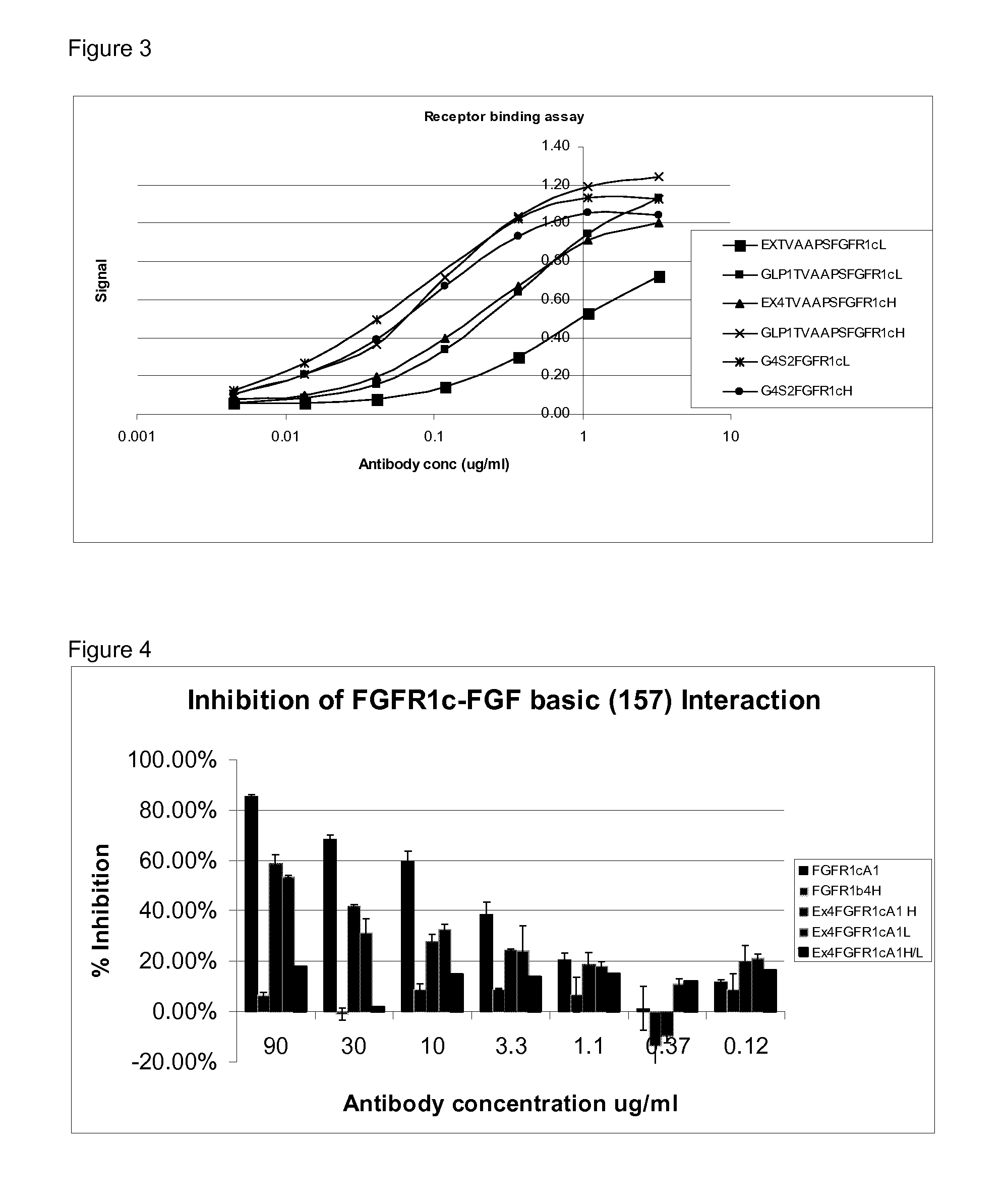
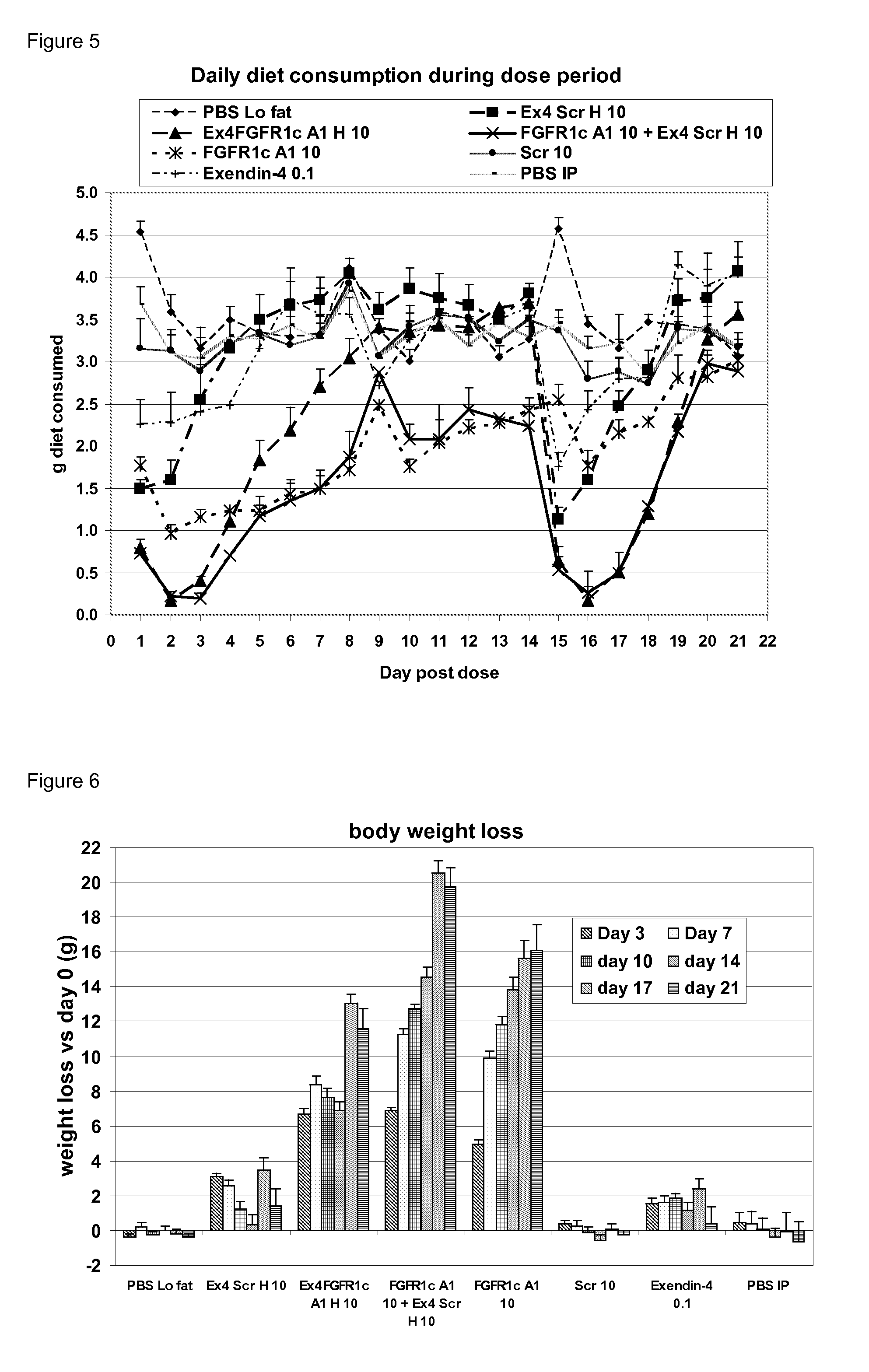
Fgfr1c antibody combinations
InactiveUS20120058116A1Lose weightAntibody mimetics/scaffoldsMetabolism disorderAgonist peptideAntigen binding
The invention relates to combinations of FGFR1c antagonists with agonist peptides and provide dual targeting proteins which bind to FEFR1c comprising an antigen binding protein which is capable of binding to FGFR1c and which is linked to one or more agonist peptides, methods of making such constructs and uses thereof, particularly in treating obesity.
Owner:GLAXO GROUP LTD
Y4 selective receptor agonists for therapeutic interventions
Y4 receptor agonist peptide selected from the group consisting of: [Ala30]PP2-36, [Thr30]PP2-36, Asn30]PP2-36, [Gln30]PP2-36, [Glu10]PP2-36, [Glu10,Leu17,Thr30]PP2-36, [Nle17,Nle30]PP2-36, [Glu10,Nle17,Nle30]PP2-36, their PP1-36 equivalents, and analogues and derivatives thereof as described in the specification, are selective agonists of the Y4 receptor relative to the Y1 and Y2 receptors, and are useful in the treatment, for example, of obesity and overweight, and conditions in which these are considered contributory factors, and in the treatment of diarrhoea and intestinal hypersecretion.
Owner:7TM PHARM AS
Formulations of guanylate cyclase C agonists and methods of use
Owner:BAUSCH HEALTH IRELAND LTD
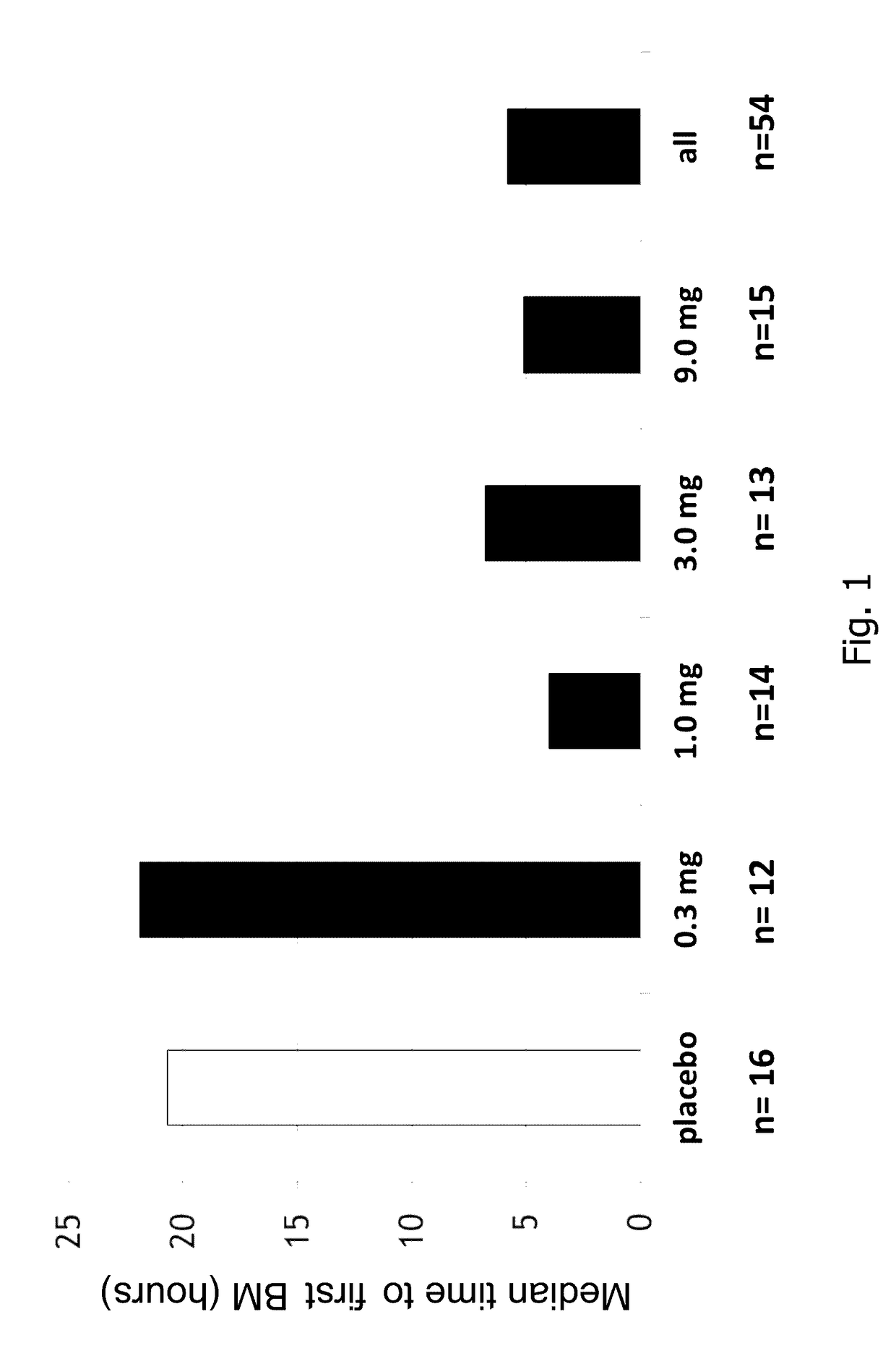
Par4 agonist peptides
ActiveUS20130289238A1Improve biological activityCell receptors/surface-antigens/surface-determinantsPeptide/protein ingredientsAgonist peptidePeptide
Owner:BRISTOL MYERS SQUIBB CO
Pegylated glucagon and glp-1 co-agonists for the treatment of obesity
InactiveUS20160017016A1Improve efficacyImprove solubilityPeptide/protein ingredientsMetabolism disorderAgonist peptideObesity
Owner:MEDIMMUNE LTD
Glucagon and glp-1 co-agonists for the treatment of obesity
ActiveUS20190185537A1Reduces fructosamine levelLose weightPeptide/protein ingredientsMetabolism disorderDiabetes mellitusAgonist peptide
Owner:MEDIMMUNE LTD
Inhibition of tumor angiogenesis by combination of thrombospondin-1 and inhibitors of vascular endothelial growth factor
InactiveUS20070020234A1Prevent and inhibitSuppression problemOrganic active ingredientsPeptide/protein ingredientsEctopic expressionAgonist peptide
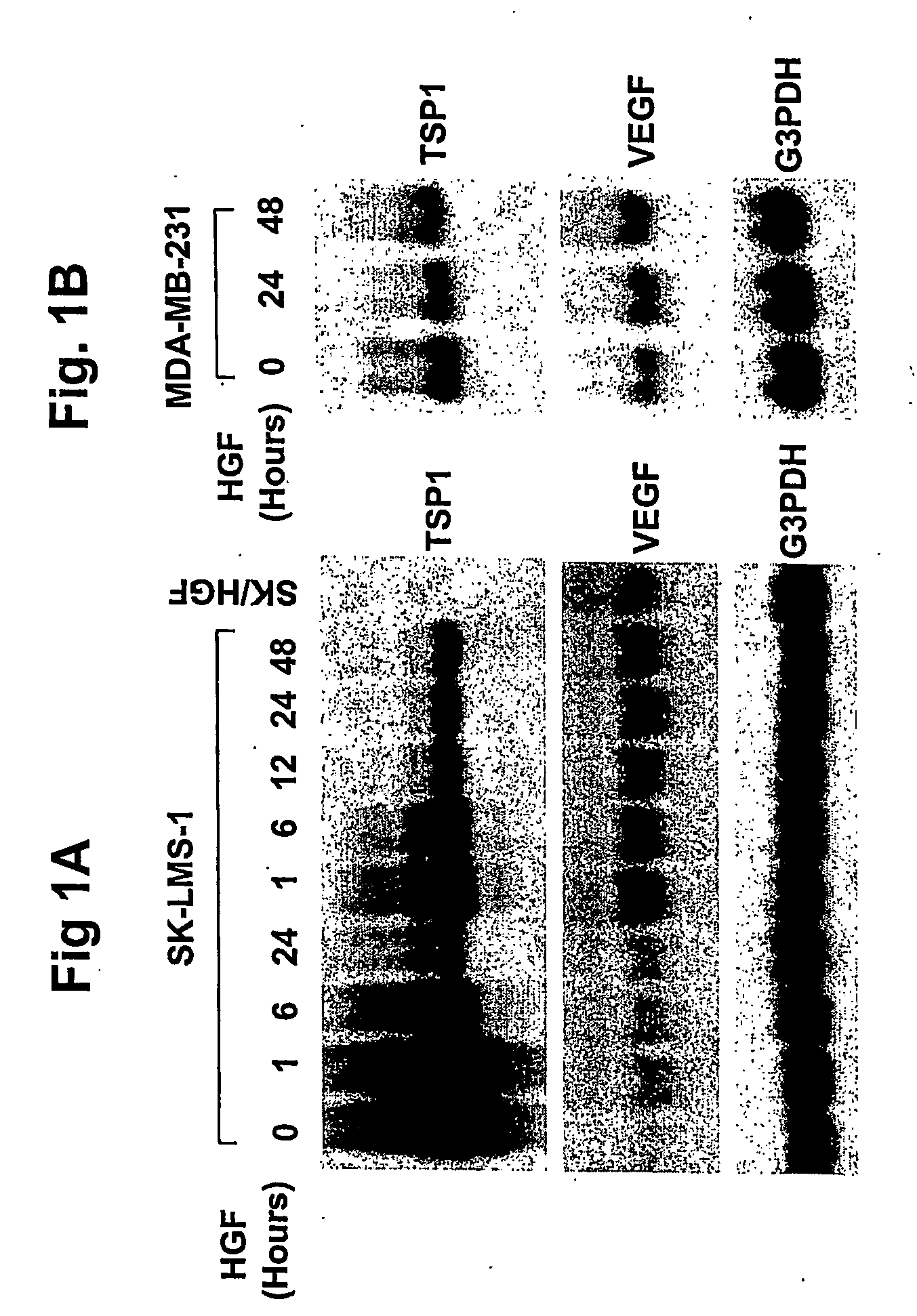
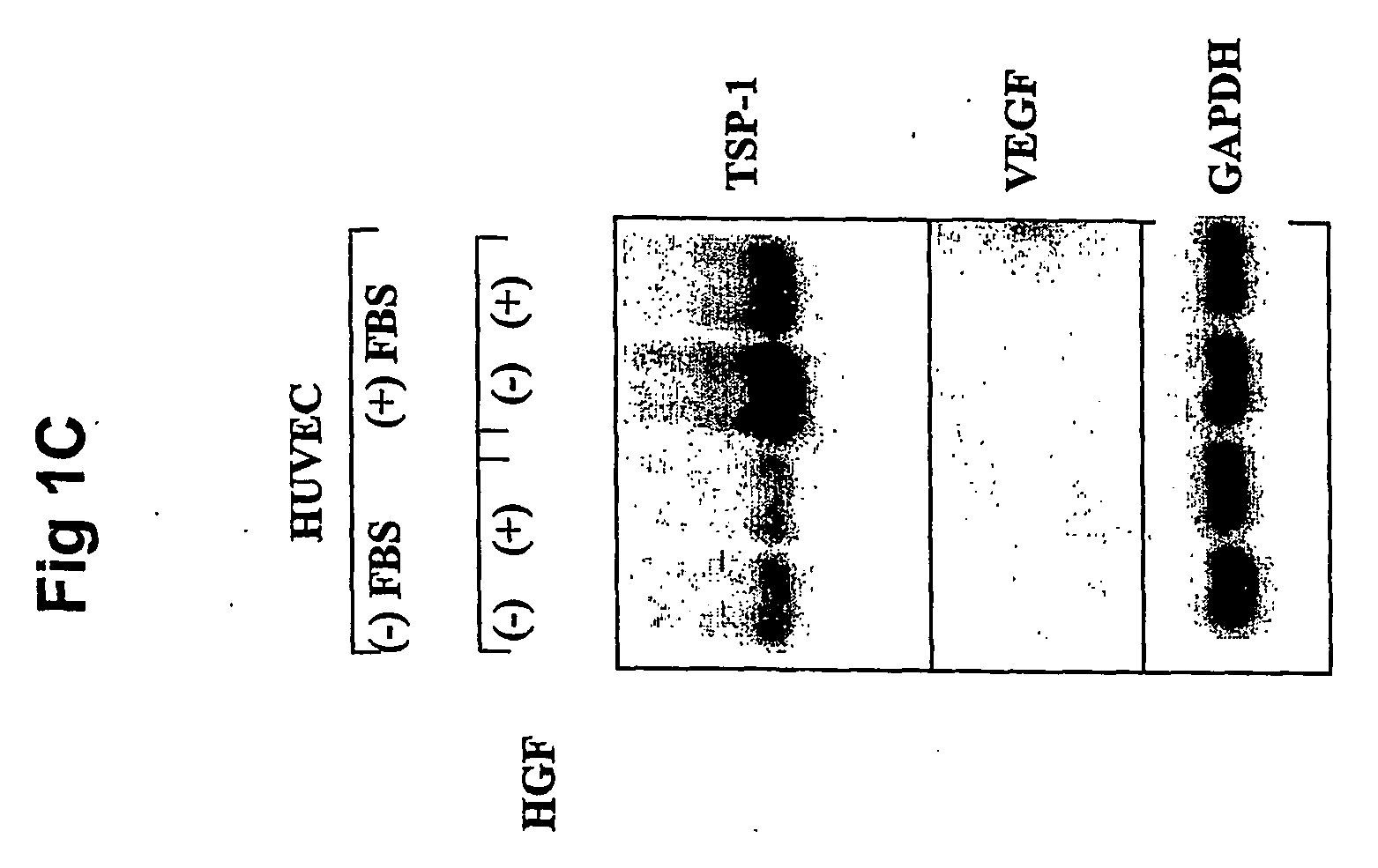
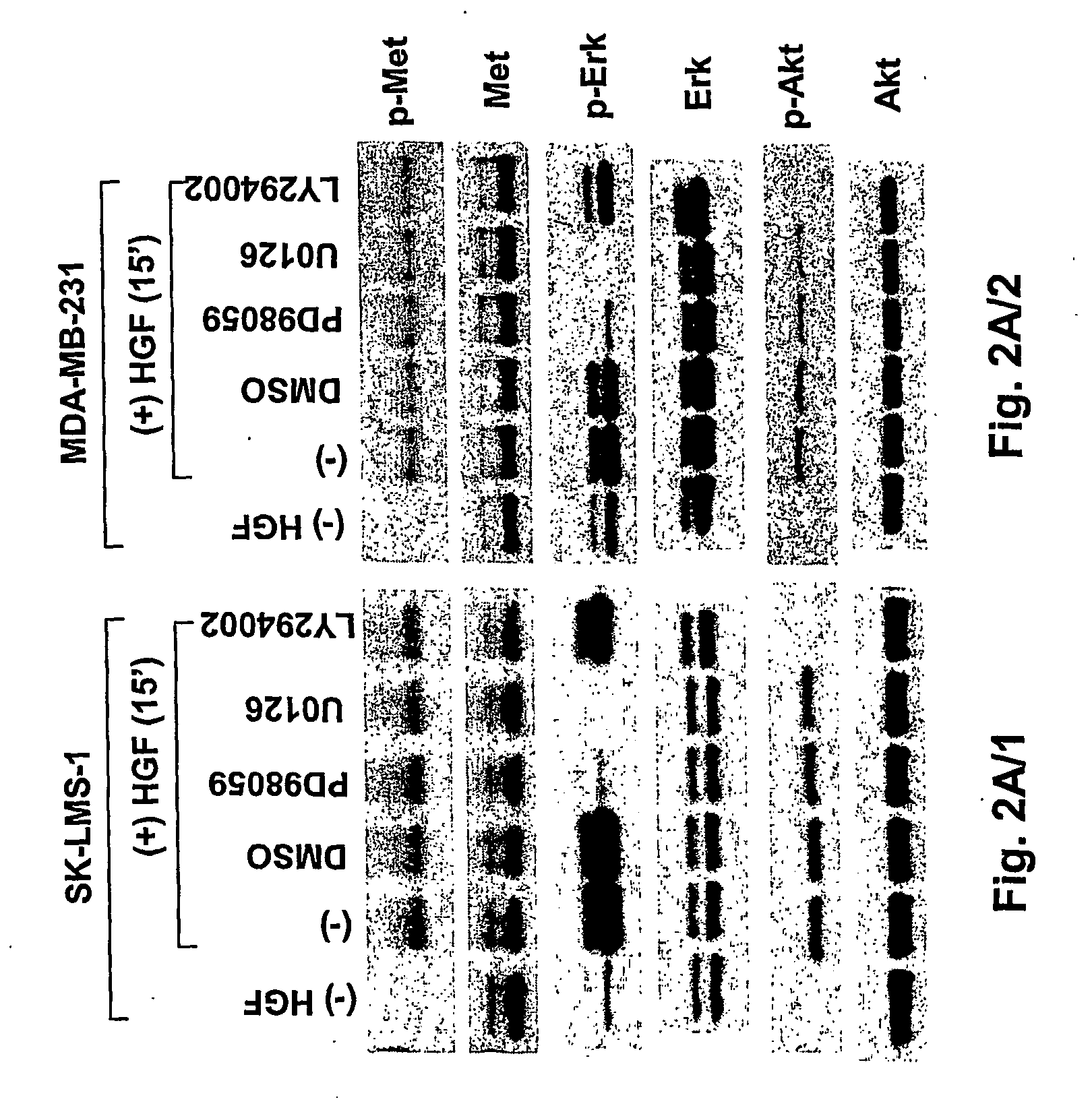
Hepatocyte growth factor / scatter factor (HGF / SF), acting through the Met receptor, plays an important role in most human solid tumors and inappropriate expression of this ligand-receptor pair is often associated with poor prognosis. The molecular basis for the malignant activity imparted by signaling of HGF / SF-Met in cancer cells has been attributed to its mitogenic and invasive properties. However, HGF / SF also induces angiogenesis, but the signaling mechanism has not been understood, nor has this activity been directly associated with HGF / SF-Met mediated tumorigenesis. HGF / SF induces expression in vitro of VEGF, a key agonist of tumor angiogenesis. By contrast, thrombospondin-1 (TSP-1) is a negative regulator of angiogenesis. This application discloses that, in the very same tumor cells, in addition to inducing VEGF expression, HGF / SF dramatically down regulates TSP-1 expression. TSP shut off plays an important, extrinsic role in HGF / SF-mediated tumor development, as ectopic expression of TSP-1 markedly inhibited tumor formation through the suppression of angiogenesis. While VEGF induced expression is sensitive to inhibitors of several pathways, including MAP kinase, PI3 kinase and Stat3, TSP-1 shut off by HGF / SF is prevented solely by inhibiting MAP kinase activation. Thus HGF / SF is a “switch” for turning on angiogenesis. TSP-1 is a useful antagonist to tumor angiogenesis, and therefore TSP-1 and agonist peptides and mimics, as well as inducers of TSP-1, have therapeutic value when used in conjunction with inhibitors of VEGF.
Owner:VAN ANDEL RES INST
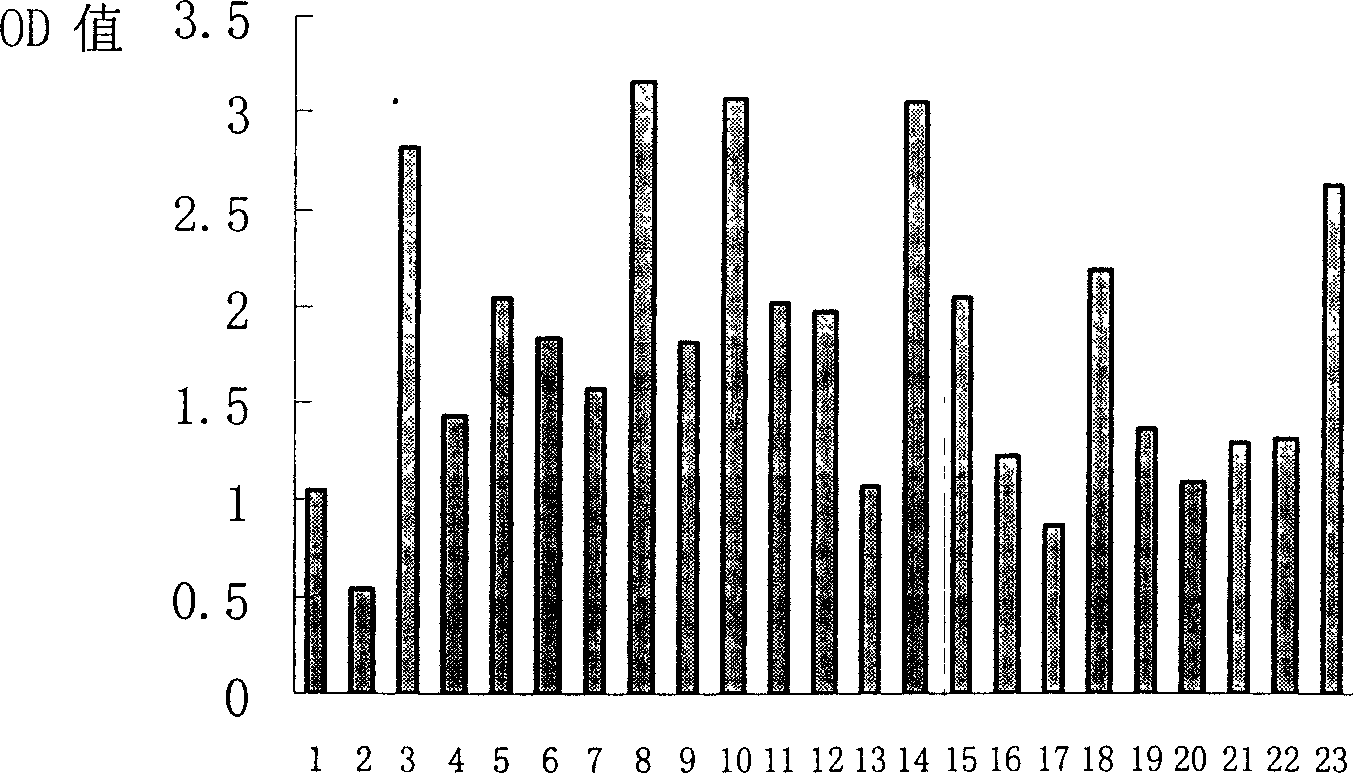
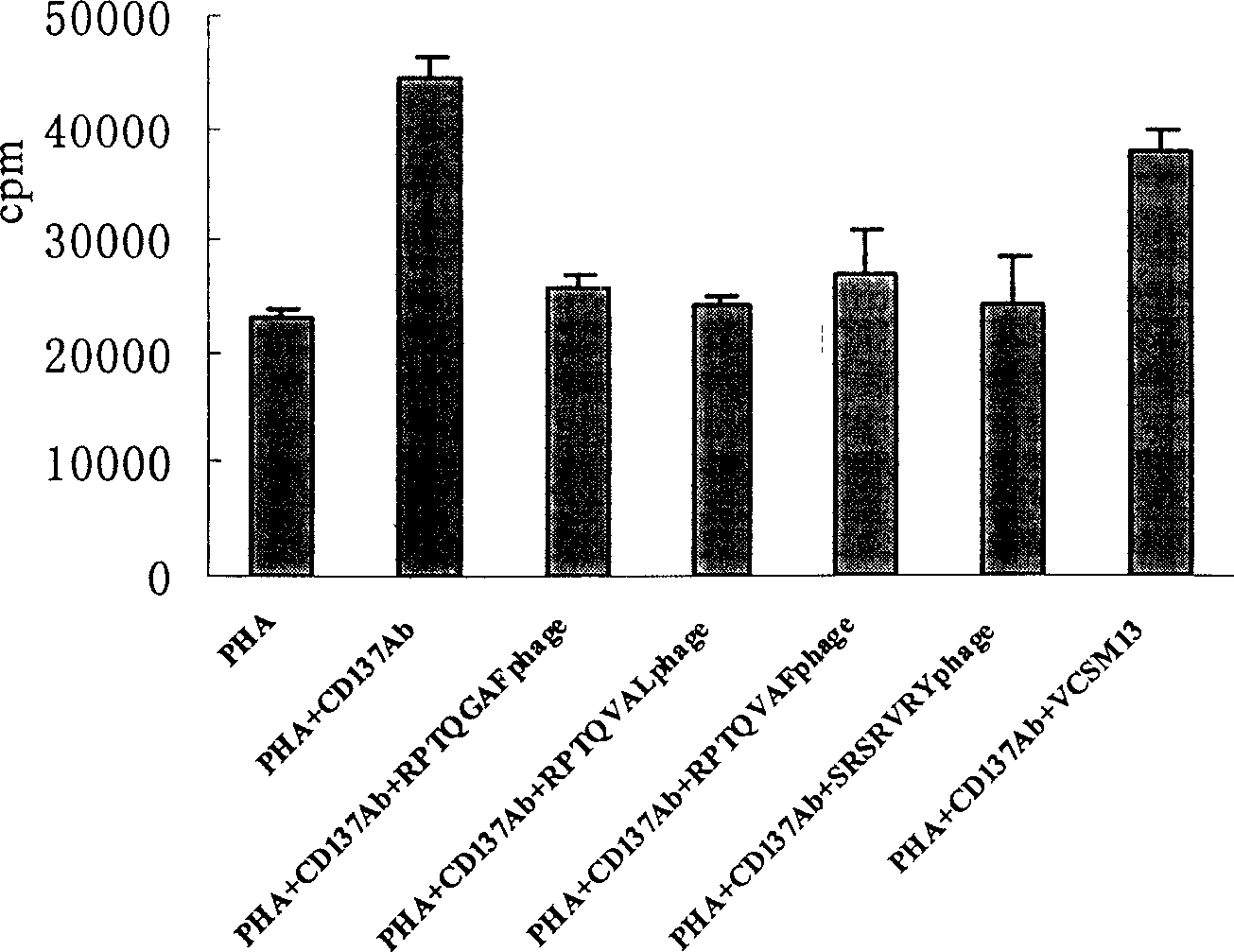
Polypeptide combined with T cell surface co-stimulation molecule CD137 and its use
The present invention discloses one group of polypeptide combined with T cell surface co-stimulating molecule CD137 with the amino acid sequence shown in SEQ ID No. 1 to SEQ ID No. 4; and the application of the polypeptides combined with T cell surface co-stimulating molecule CD137 in preparing medicine for treating autoimmune disease, tumor graft rejection, preparing medicine for activating or blocking CD137-CD137L passage, and developing and preparing CD137-CD137L agonist peptide and peptoid.
Owner:SHANDONG UNIV
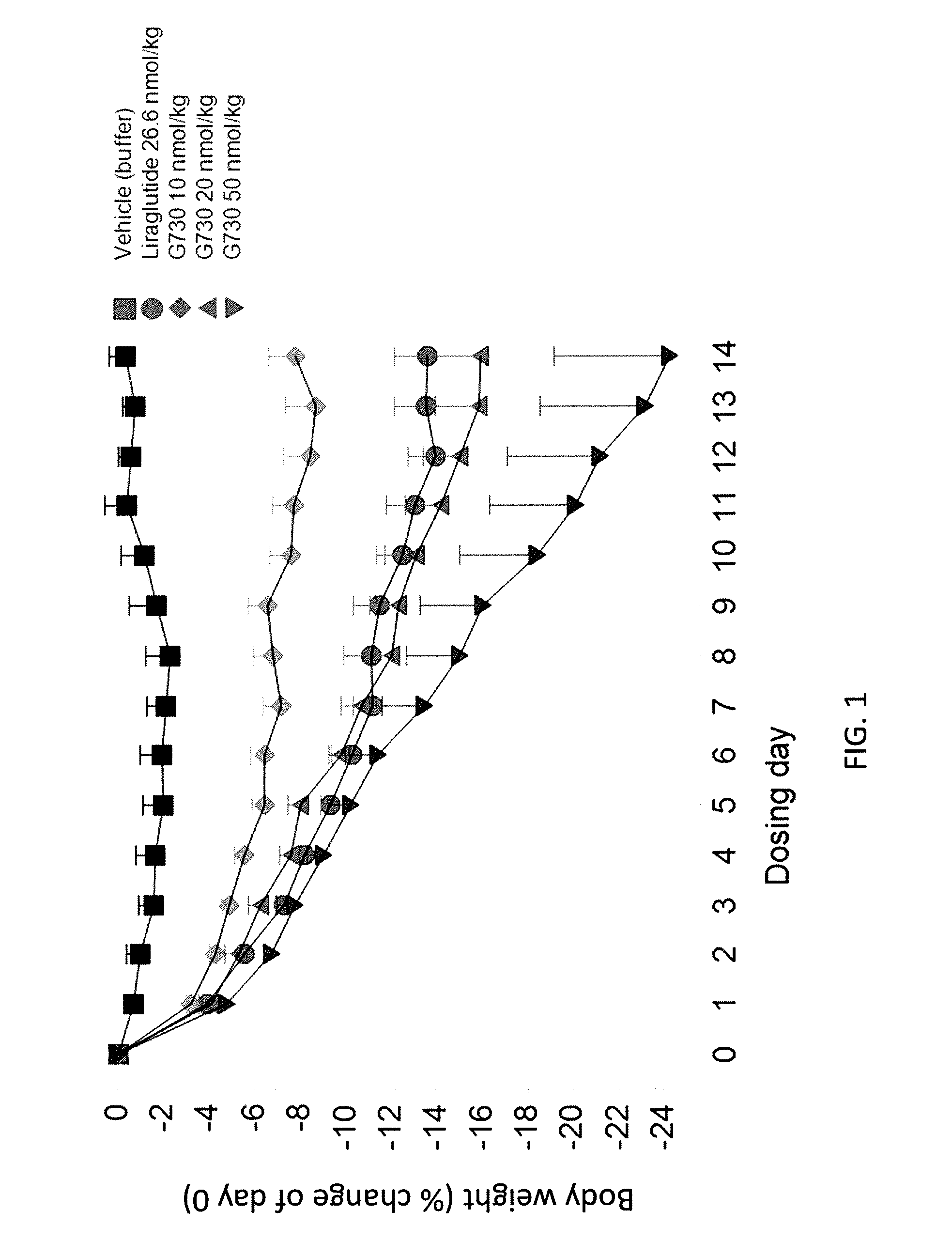
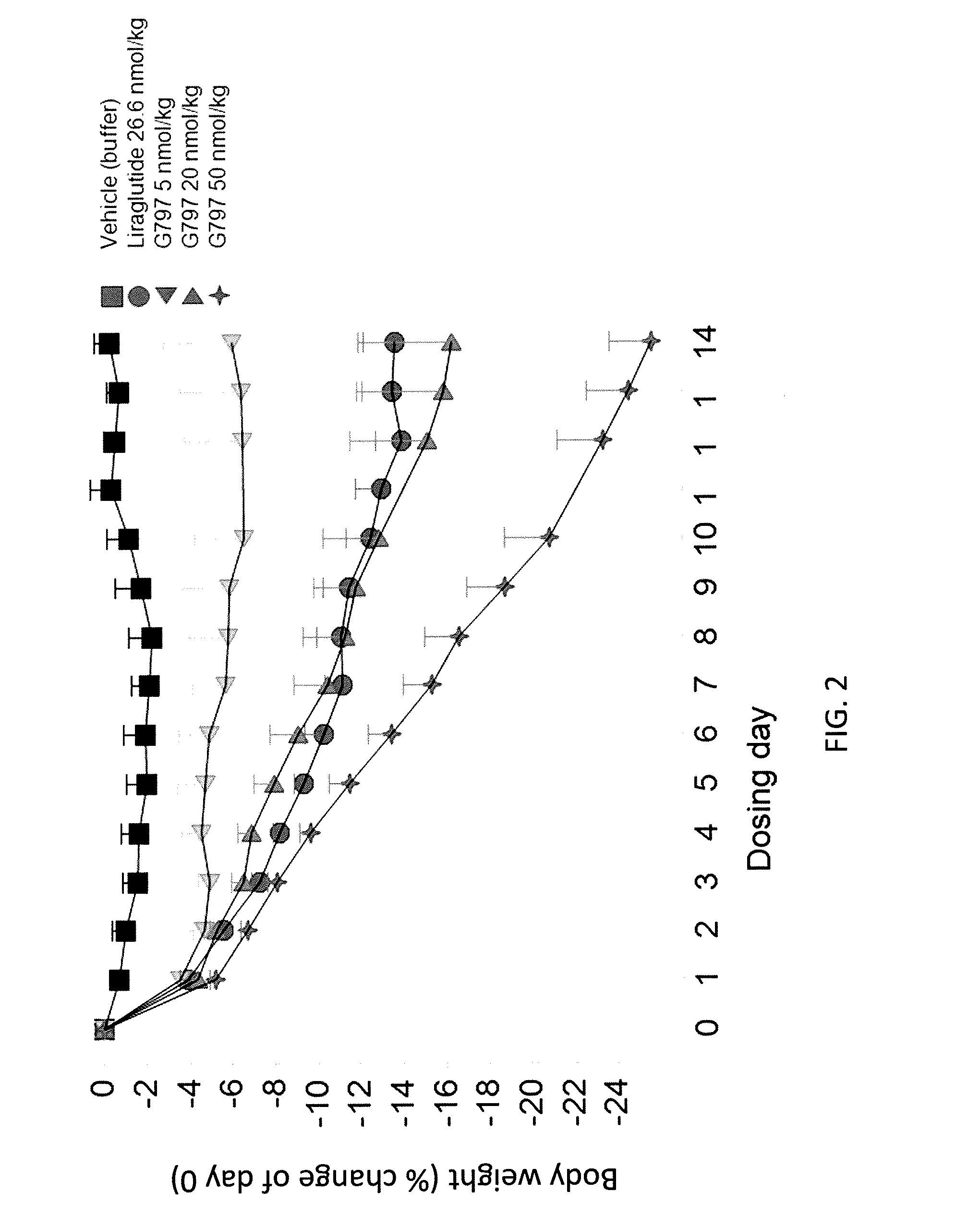
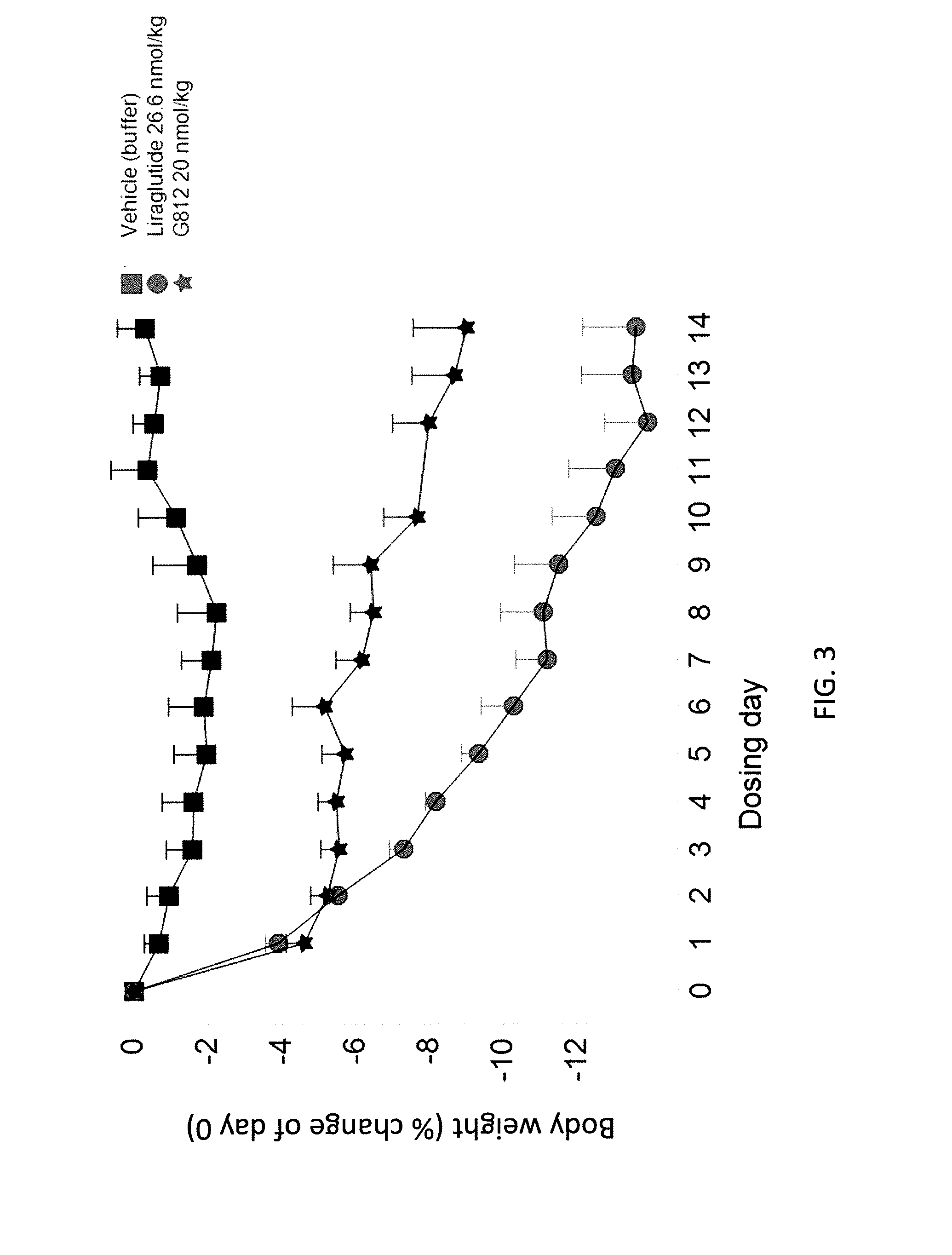
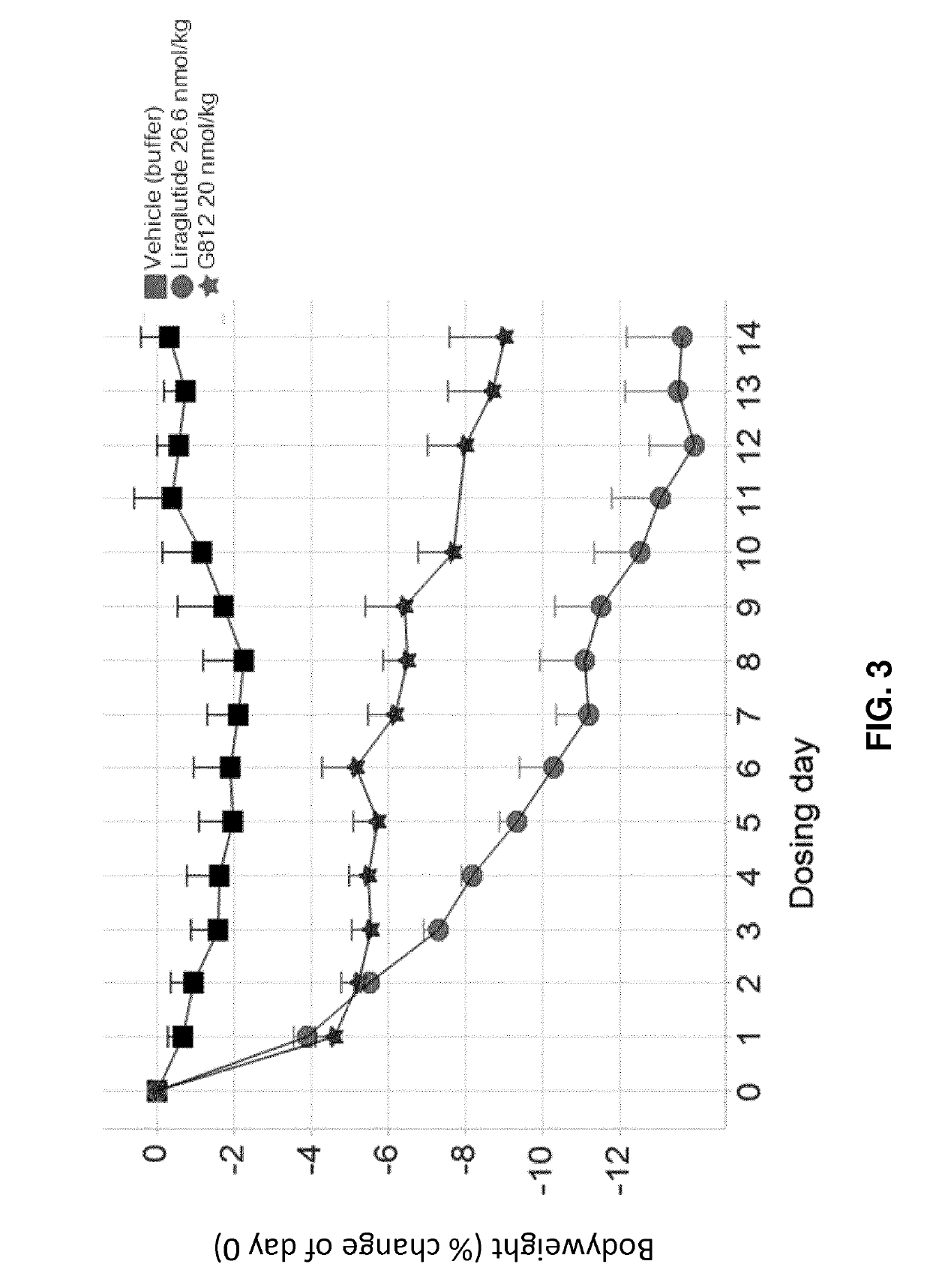
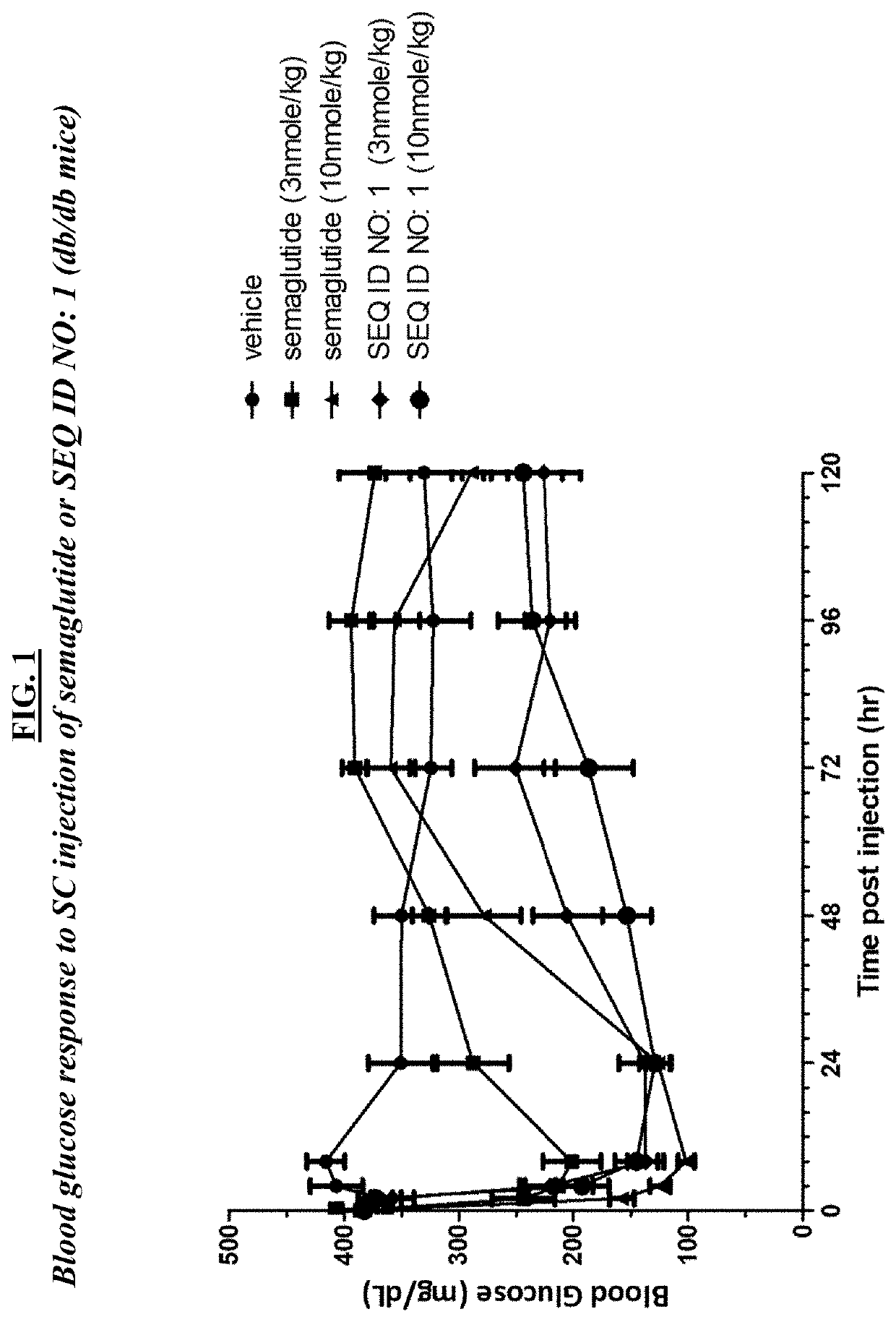
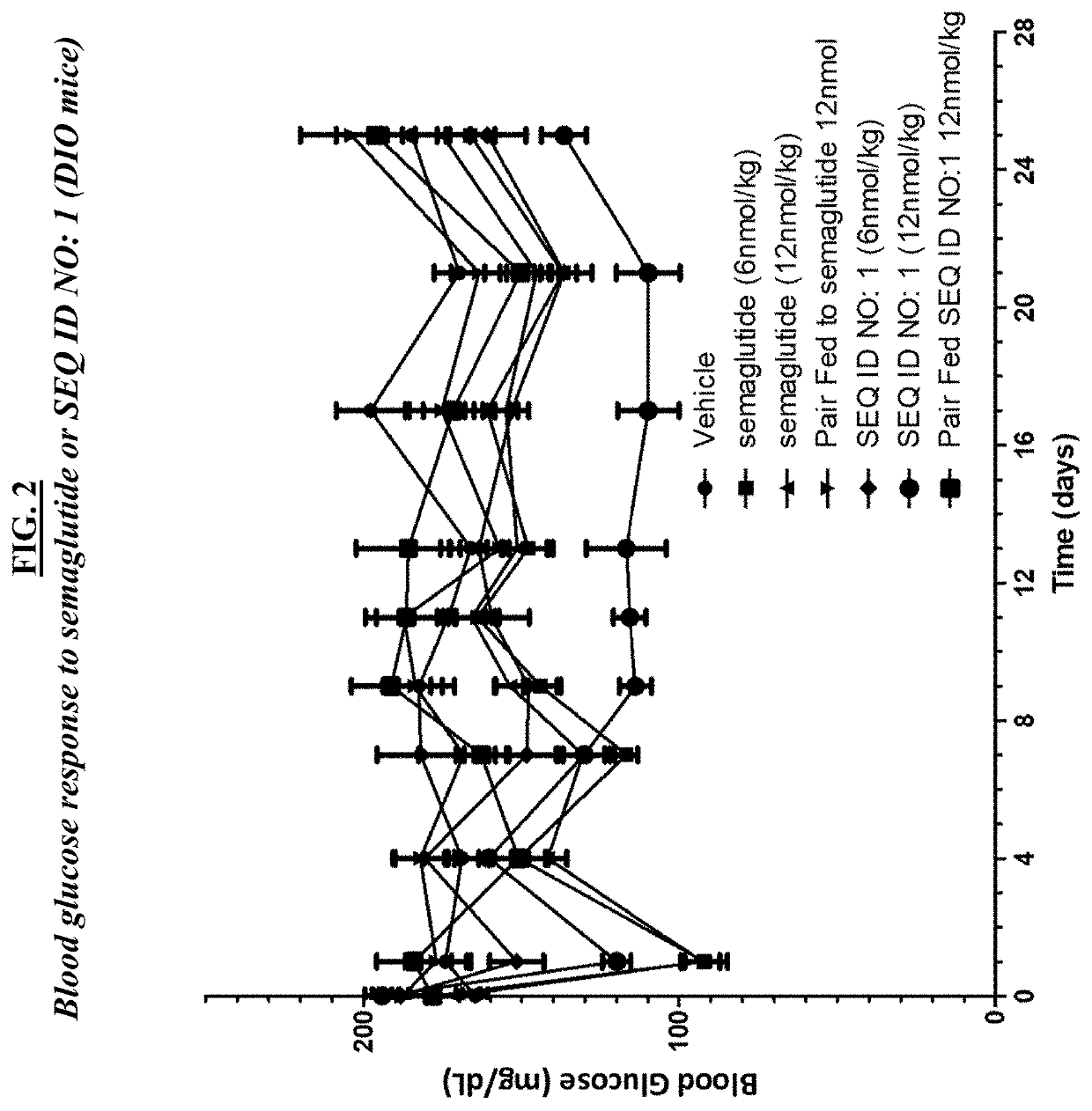
GLP-1R and GCGR Agonists, Formulations, and Methods of Use
PendingUS20210290732A1Treated conditionReduces and eliminates dosage-related adverse eventPeptide/protein ingredientsMetabolism disorderActive agentAgonist peptide
This disclosure relates to the field of GLP-1R and GCGR agonists, formulations, and methods of using the same, including but not limited to dual agonist peptides of any of SEQ ID NOS. 1-10 or 12-27 conjugated to a non-ionic glycolipid surfactant.
Owner:SPITFIRE PHARM LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com