Formulations of guanylate cyclase c agonists and methods of use
a technology of guanylate cyclase and agonists, applied in the field of new formulations, can solve the problems of side effects of conventional oral formulations intended to treat ibd, for instance, and achieve the effects of reducing or eliminating degradation and aggregation, reducing or eliminating side effects, and reducing the exposure of gcc agonists
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis and Purification of GCC Agonist Peptides
[0218]The GCC agonist peptides were synthesized using standard methods for solid-phase peptide synthesis. Either a Boc / Bzl or Fmoc / tBu protecting group strategy was selected depending upon the scale of the peptide to be produced. In the case of smaller quantities, it is possible to get the desired product using an Fmoc / tBu protocol, but for larger quantities (1 g or more), Boc / Bzl is superior.
[0219]In each case the GCC agonist peptide was started by either using a pre-loaded Wang (Fmoc) or Merrifield (Boc) or Pam (Boc) resin. For products with C-terminal Leu, Fmoc-Leu-Wang (D-1115) or Boc-Leu-Pam resin (D-1230) or Boc-Leu-Merrifield (D-1030) Thus, for peptides containing the C-terminal d-Leu, the resin was Fmoc-dLeu-Wang Resin (D-2535) and Boc-dLeu-Merrifield, Boc-dLeu-Pam-Resin(Bachem Product D-1230 and D-1590, respectively) (SP-332 and related analogs). For peptides produced as C-terminal amides, a resin with Ramage linker (Bachem ...
example 2
In Vitro Biological and Chemical Stability of SP-304 after Incubation in Simulated Gastric Fluid (SGF)
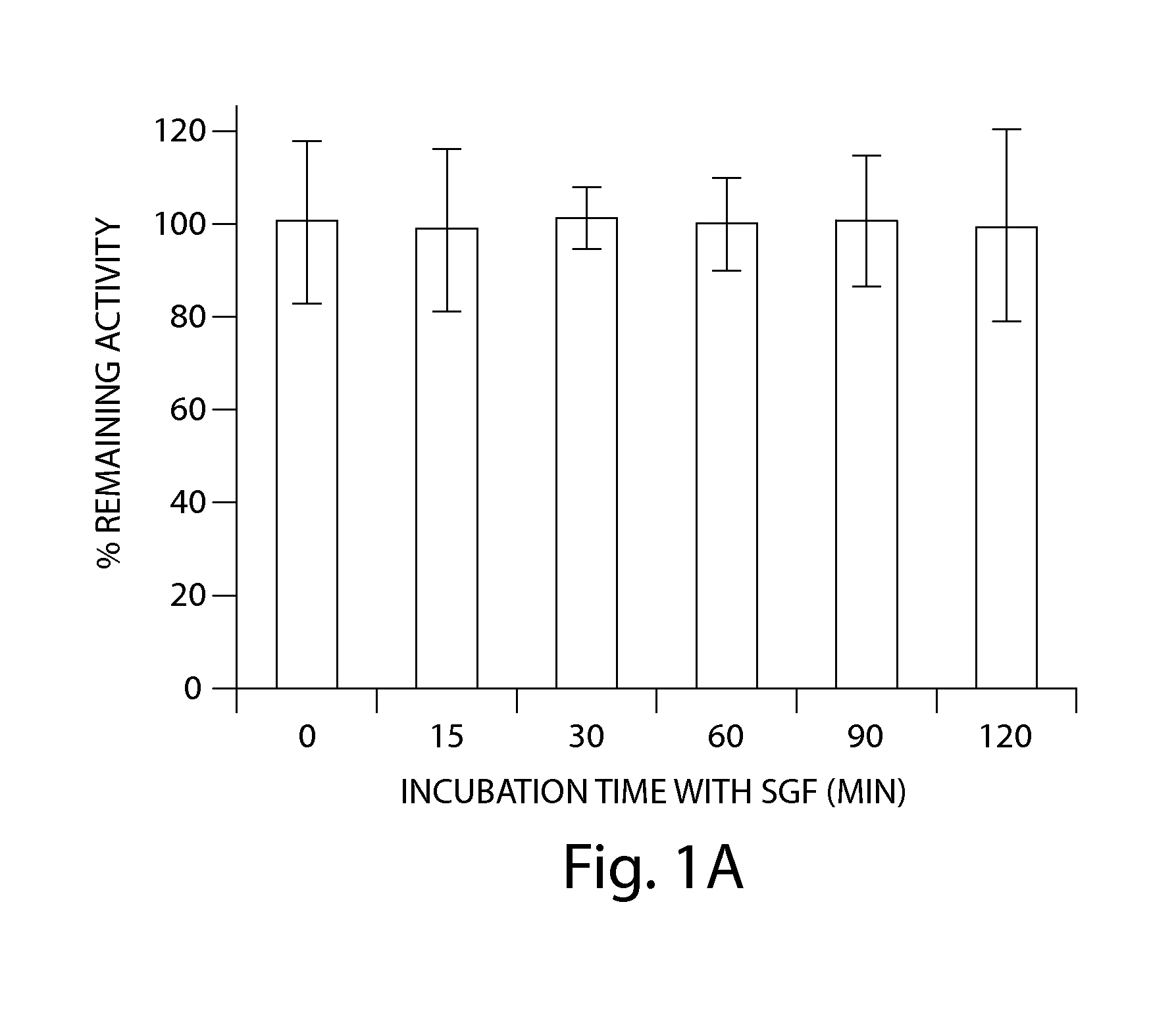
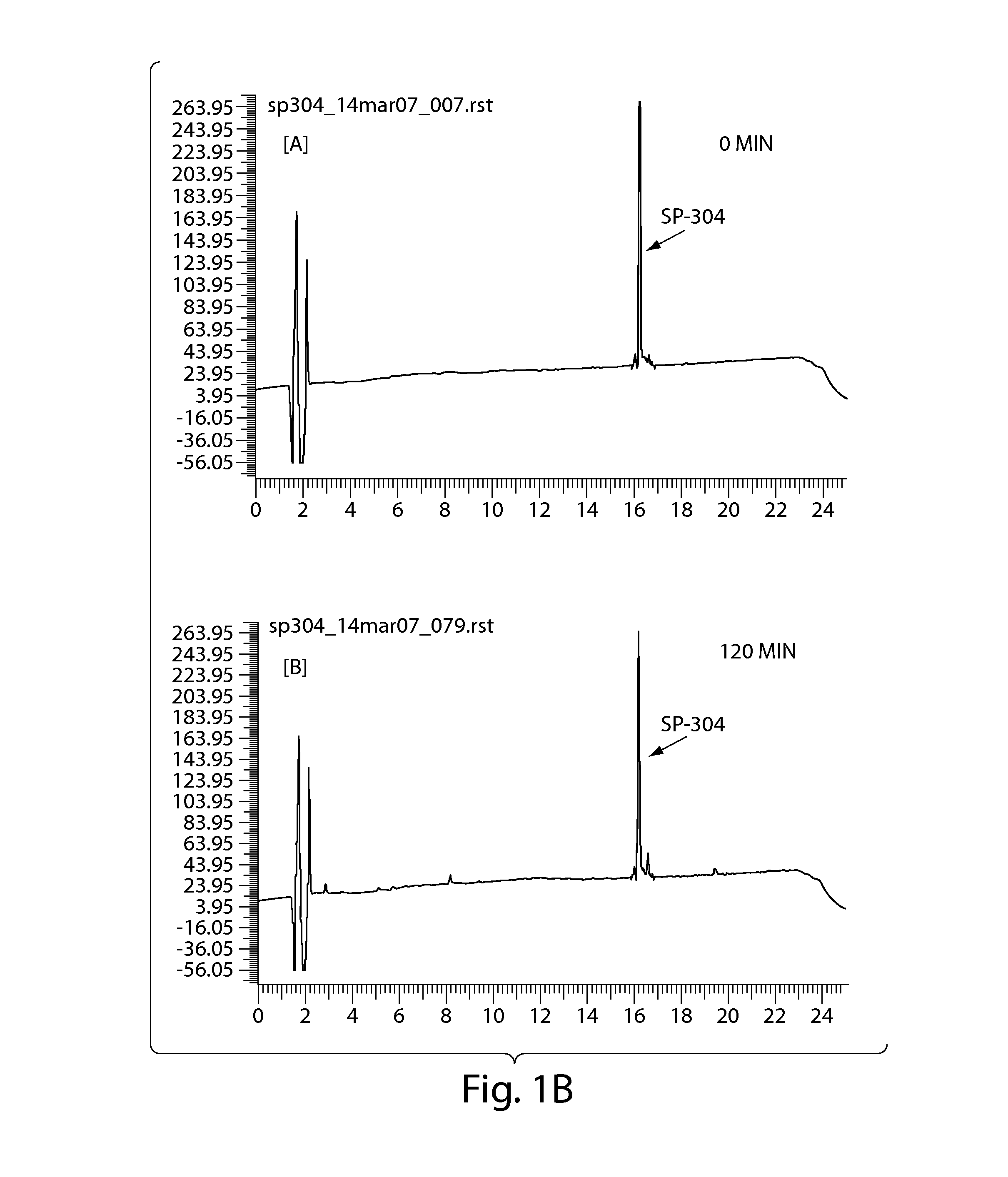
[0231]The stability of SP-304 in the presence of simulated gastric fluid (SGF) was determined by biological activity measurements and HPLC analyses (FIGS. 1A & 1B). SP-304 (final concentration of 8.5 mg / ml) was incubated in SGF (Proteose peptone (8.3 g / liter; Difco), D-Glucose (3.5 g / liter; Sigma), NaCl (2.05 g / liter; Sigma), KH2PO4 (0.6 g / liter; Sigma), CaCl2 (0.11 g / liter), KCl (0.37 g / liter; Sigma), Porcine bile (final 1× concentration 0.05 g / liter; Sigma) in PBS, Lysozyme (final 1× concentration 0.10 g / liter; Sigma) in PBS, Pepsin (final 1× concentration 0.0133 g / liter; Sigma) in PBS). SGF was made on the day of the experiment and the pH was adjusted to 2.0±0.1 using HCl or NaOH as necessary. After the pH adjustment, SGF is filter sterilized with 0.22 μm membrane filters. SP-304 (final concentration of 8.5 mg / ml) was incubated in SGF at 37° C. for 0, 15, 30, 45, 60 and 120 min, ...
example 3
In Vitro Biological and Chemical Stability of SP-304 after Incubation in Simulated Intestinal Fluid (SIF)
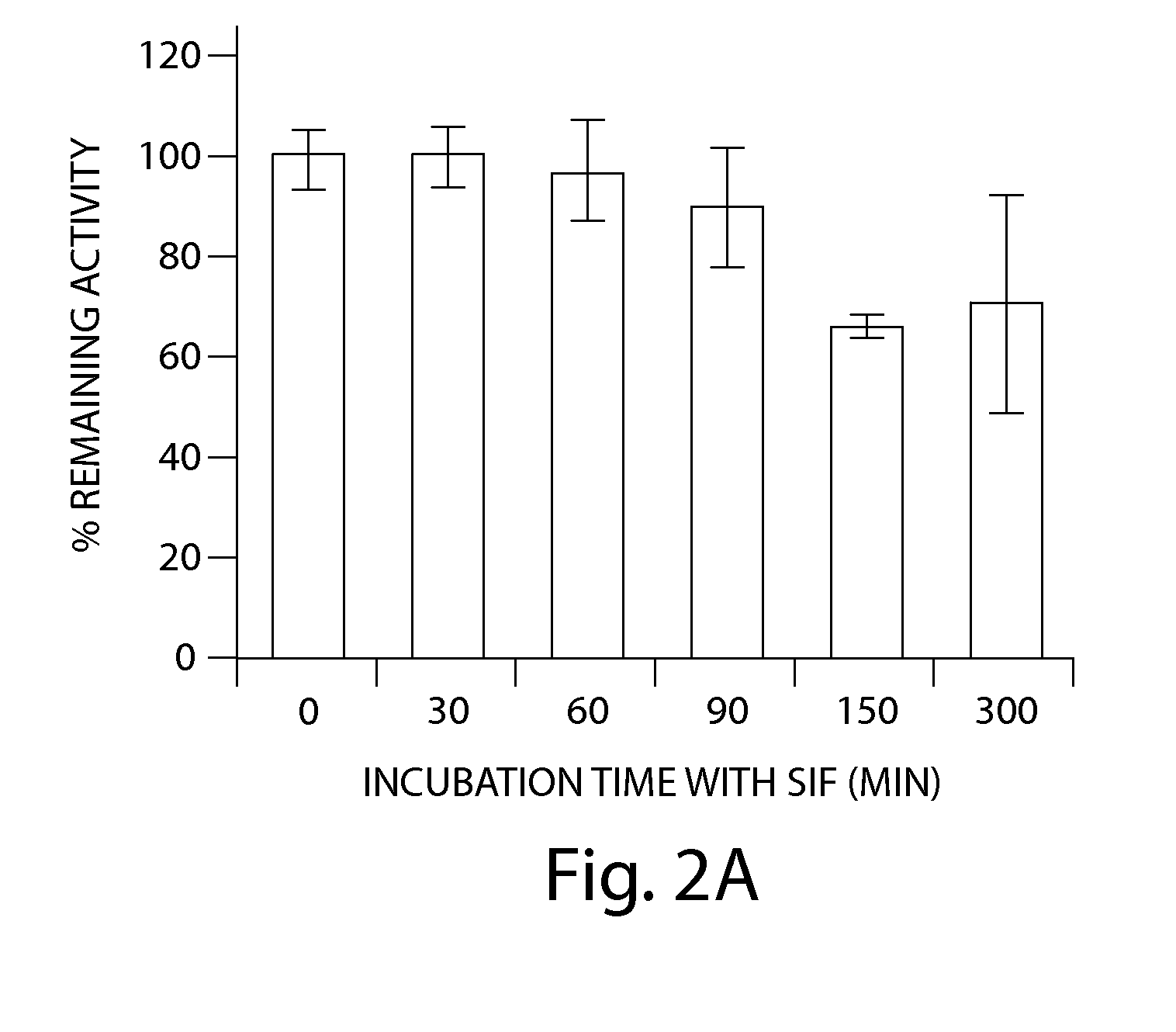
[0234]The stability of SP-304 was also evaluated after incubation with simulated intestinal fluid (SIF) by measuring its biological activity and by HPLC analyses (FIGS. 2A & 2B). SIF solution was prepared by the method as described in the United States Pharmacopoeia, 24th edition, p2236. The recipe to prepare SIF solution was as described below. The SIF solution contained NaCl (2.05 g / liter; Sigma), KH2PO4 (0.6 g / liter; Sigma), CaCl2 (0.11 g / liter), KCl (0.37 g / liter; Sigma), and Pacreatin 10 mg / ml. The pH was adjusted to 6 and the solution was filter sterilized. A solution of SP-304 (8.5 mg / ml) was incubated in SGF at 37° C. for 0, 30, 60, 90, 120, 150 and 300 min respectively, in triplicate aliquots. Following incubations, samples were removed and snap frozen with dry ice and stored in a −80° C. freezer until assayed in duplicate. FIG. 2A is a bar chart showing the ability of S...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com