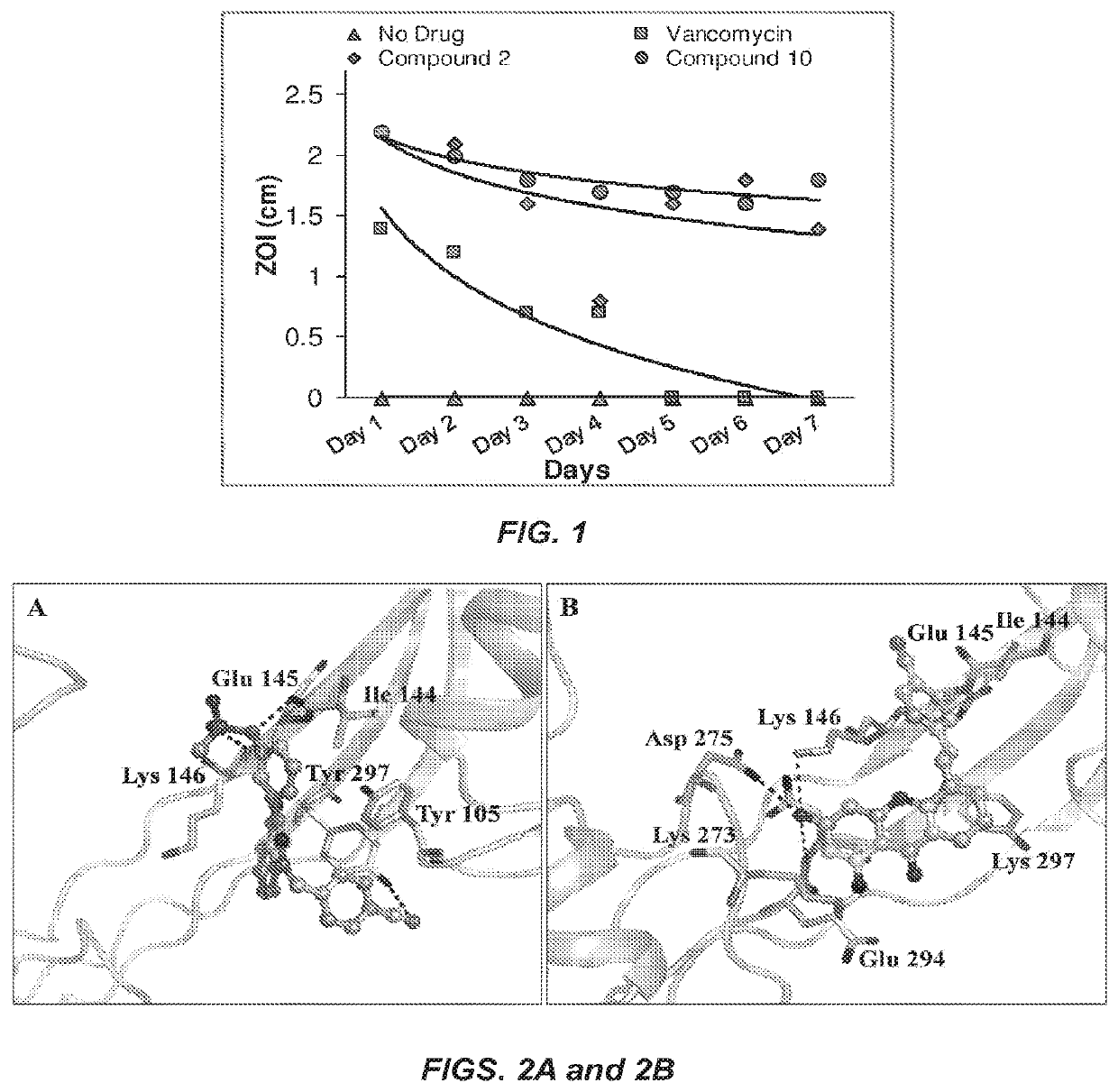
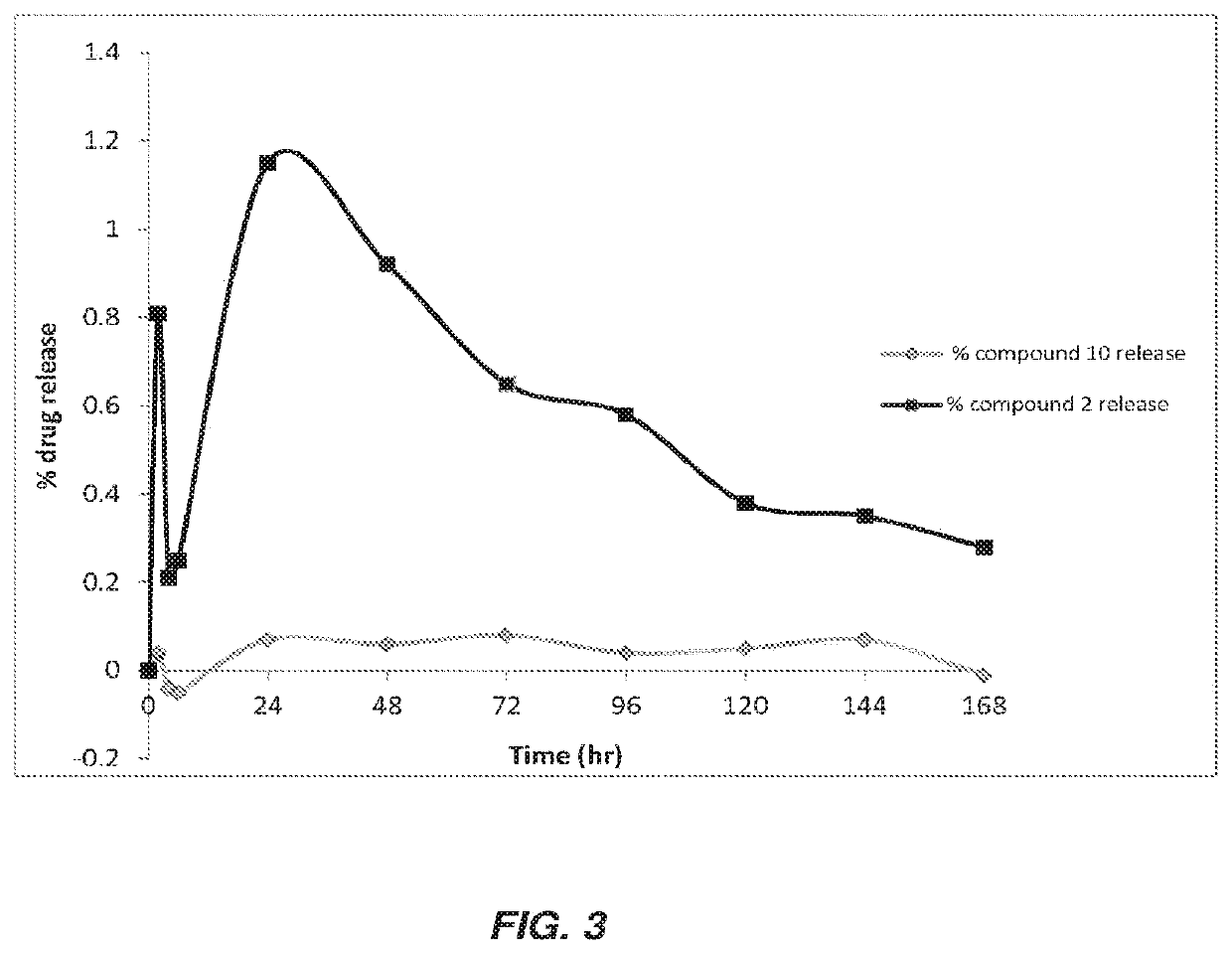
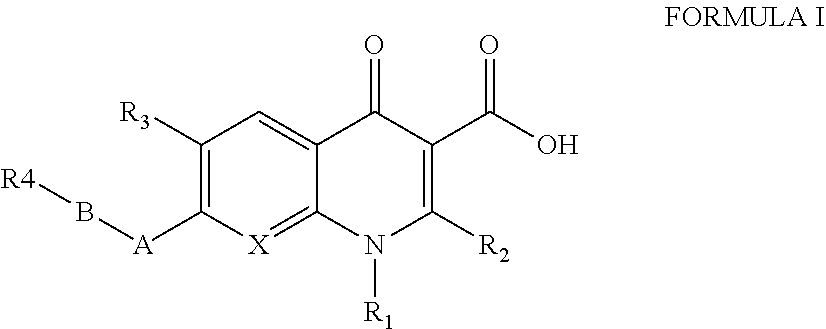
Antibacterial therapeutics and prophylactics
a technology of antibacterial therapy and prophylactics, applied in the field of new molecules, compositions and formulations for the treatment of bacterial infections, can solve the problems of reducing the permeability or uptake of antibiotics, affecting and affecting the permeability of antibiotics, so as to minimize nephrotoxicity, ototoxicity and gastrointestinal side effects, the effect of improving the delivery of antibiotics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
of Compound 6
[0345]
2-Chloro-N-(5-nitrothiazol-2-yl)-acetamide (A)
[0346]To a stirred solution of 5-nitrothiazol-2-amine (2 g, 13.80 mmol) and triethylamine (2.85 ml, 20.60 mmol) in acetonitrile, chloroacetyl chloride (1.44 ml, 17.80 mmol) was added slowly at 0° C., and the reaction mixture was stirred at room temperature for four hours. Then solvent was evaporated at reduced pressure and extracted with ethyl acetate. The organic layer was washed with 1 N HCl, brine and finally dried over sodium sulphate to obtain the compound A which was directly used for next reaction without further purification (2.6 g, 85%). 1H NMR (CDCl3): δ 8.29 (s, 1H, ArH), 4.28 (s, 1H, CH2).
6-Fluoro-1-methyl-7-(4-(2-((5-nitrothiazol-2-yl)amino)-2-oxoethyl)piperazin-1-yl)-4-oxo-1H,4H-[1,3]-thiazeto[3,2-a]quinoline-3-carboxylic acid (6)
[0347]To a stirred solution of 6-fluoro-1-methyl-4-oxo-7-(piperazin-1-yl)-1H,4H-[1,3]thiazeto[3,2-a]quinoline-3-carboxylic acid (1.70 g, 4.87 mmol) and triethylamine (1.33 ml, 9....
example 3
of Compound 10
[0348]
(5-Nitrofuran-2-yl)methanol (A)
[0349]To a solution 5-nitrofuran-2-carbaldehyde (794 mg, 5.63 mmol) in dry methanol (10 ml), sodium borohydride (320 mg, 8.44 mmol) was added in portions at 0° C. Then the reaction mixture was stirred at room temperature for another 1 h. After completion, the reaction mixture was acidified with 3 N HCl and extracted with ethyl acetate. Organic layer was washed with brine, dried over sodium sulphate and finally solvent evaporated under vacuum to obtain the compound A. This was directly used for the next step without further purification (670 mg, 83%).
2-(Bromomethyl)-5-nitrofuran (B)
[0350]To a stirred solution of A (928 mg, 6.50 mmol) in DCM (20 ml), phosphorus tribromide (0.80 ml, 8.40 mmol) was added slowly at 0° C., and reaction mixture was stirred at room temperature for one hour. On completion, the reaction mixture was poured into crushed ice, neutralized with sodium bicarbonate solution and extracted with DCM, which on evaporati...
example 4
of Compound 11
[0352]
5-Nitrofuran-2-carbonyl chloride (A)
[0353]To an ice cold solution of 5-nitrofuran-2-carboxylic acid (450 mg, 2.90 mmol) in DCM (10 ml) oxalyl chloride (2.50 L, 29 mmol) was added followed by addition of catalytic amount of DMF at 0° C., and the reaction mixture was allowed to stir for 3 h at room temperature. On completion, the solvent was evaporated under reduced pressure to obtain the acid chloride A with a quantitative yield (498 mg).
6-Fluoro-1-methyl-7-(4-(5-nitrofuran-2-carbonyl)piperazin-1-yl)-4-oxo-1H,4H-[1,3]thiazeto[3,2-a]-quinoline-3-carboxylic acid (11)
[0354]To a stirred solution of 6-fluoro-1-methyl-4-oxo-7-(piperazin-1-yl)-1H,4H-[1,3]thiazeto[3,2-al]quinoline-3-carboxylic acid (1.2 g, 3.44 mmol) in DMF, triethylamine (0.80 ml, 5.7 mmol) was added, followed by addition of A (500 mg, 2.80 mmol) in DMF at 0° C. Then reaction mixture was stirred at room temperature for 18 h. After completion of the reaction excess ethyl acetate was added into the reactio...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap