Flex-nucleoside analogues, novel therapeutics against filoviruses and flaviviruses
a technology of flex-nucleosides and analogues, applied in the field of flex-nucleoside analogues, novel therapeutics against filoviruses and flaviviruses, can solve the problems of high mortality rate and severe hemorrhagic fever, and achieve the effect of retaining potency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
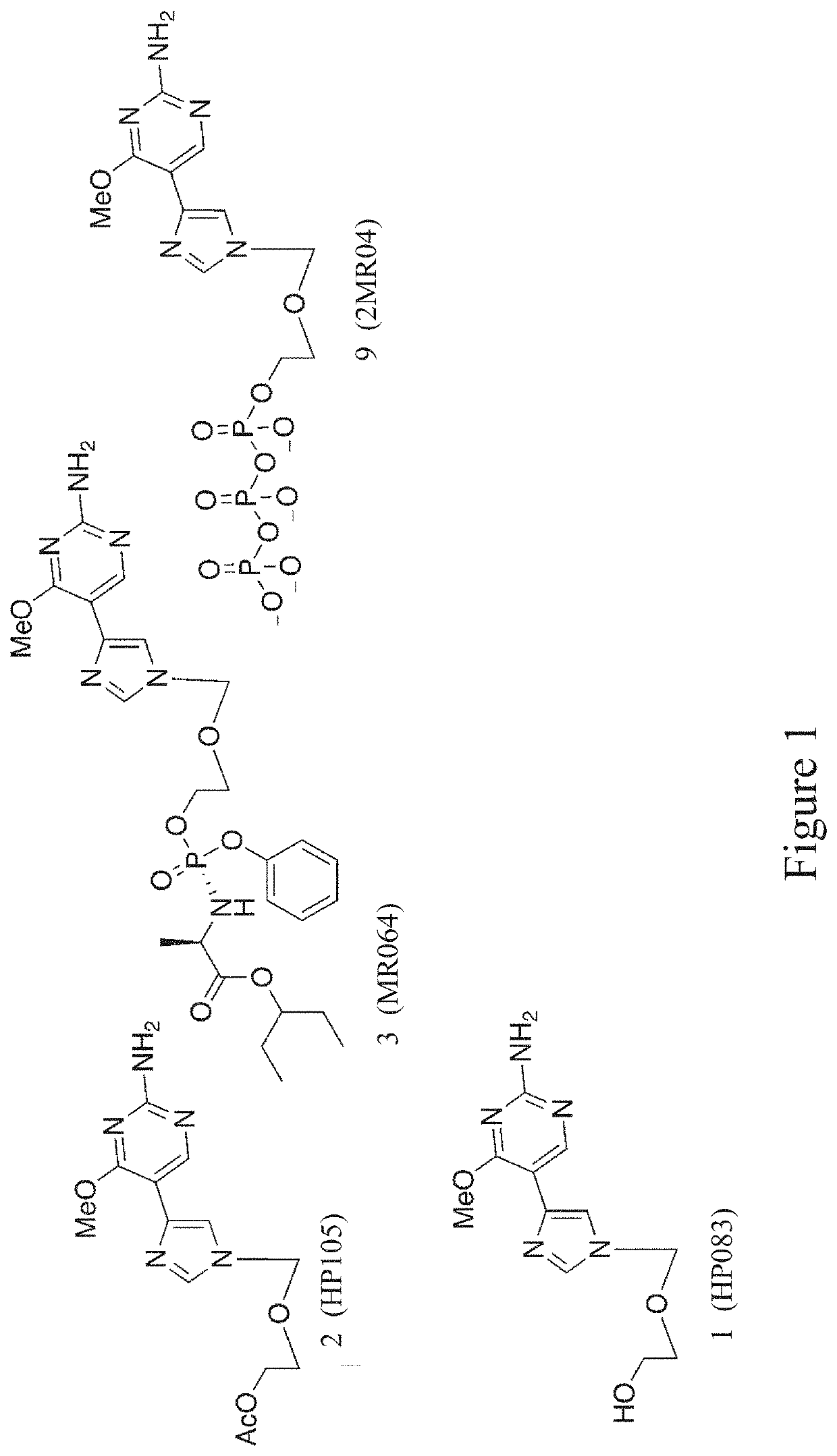
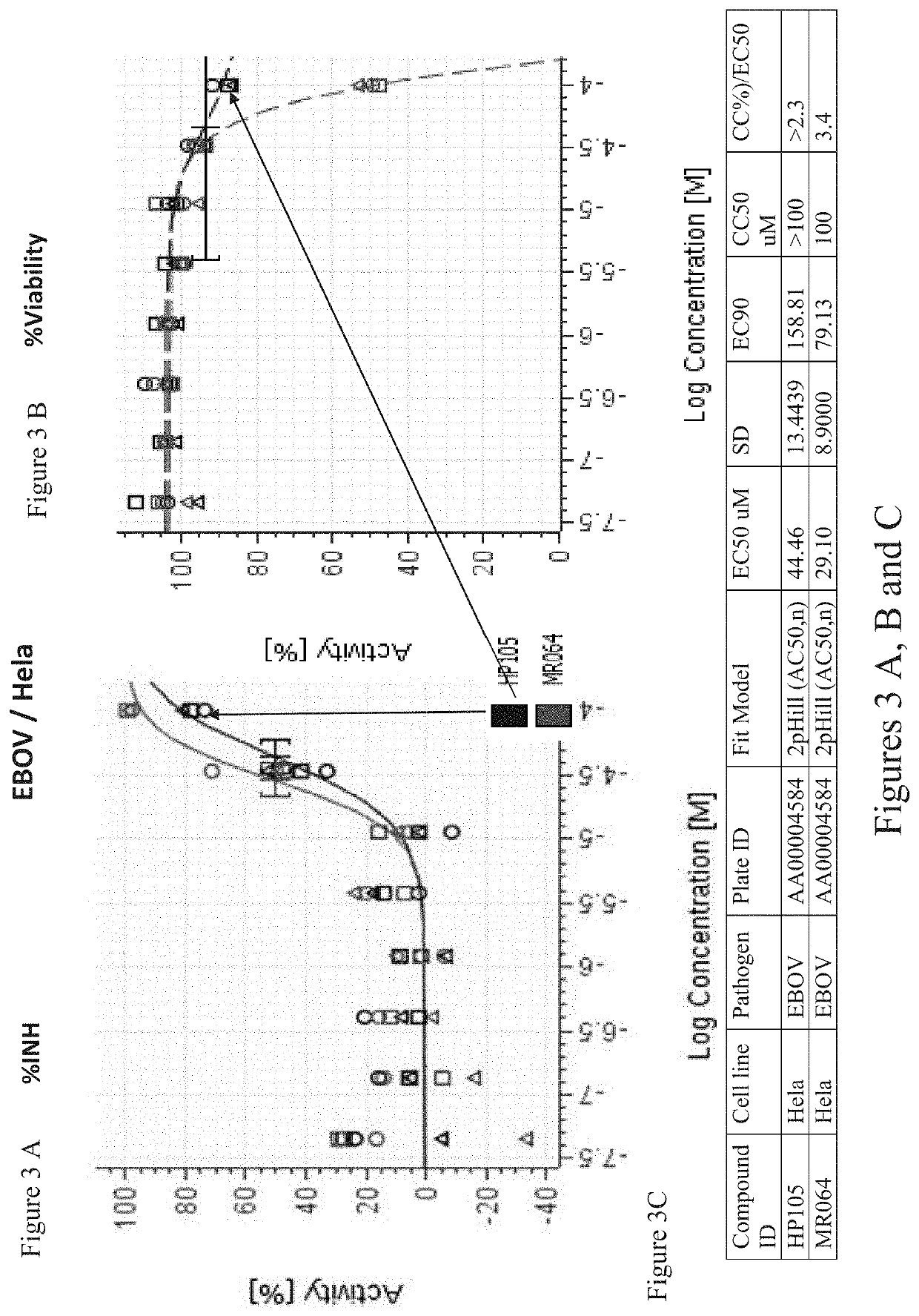
[0042]Unique nucleoside analogues have been termed ‘fleximers’ and were designed to explore how nucleobase flexibility affects the recognition, binding, and activity of nucleoside(tide) analogues. The fleximers possess a purine base scaffold in which the pyrimidine moiety is attached by a single carbon-carbon bond, rather than being ‘fused’ as is typical for the purines. These analogues are designed to retain all of the requisite purine hydrogen bonding patterns while allowing the nucleobase to explore alternative binding modes.
[0043]The present invention provides for a series of doubly flexible nucleoside analogues based on the acyclic sugar scaffold of acyclovir and the flex-base moiety found in the fleximers. The target compounds were evaluated for their antiviral potential and found to inhibit filoviruses, flaviviruses or coronaviruses.
[0044]Mammal or human hosts infected with a filovirus, flavivirus or coronavirus can be treated by administering to said mammal or human an effec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com