Process for sodium sulfide/ferrous sulfate treatment of hexavalent chromium and other heavy metals
a technology of sodium sulfide and ferrous ion, which is applied in the direction of water/sewage multi-stage treatment, water/sewage treatment by neutralisation, separation process, etc., can solve the problems of ferrous ion not being able to reduce cr by itself, industrial waste treatment plants downstream from electroplating facilities are generally subject to industrial wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
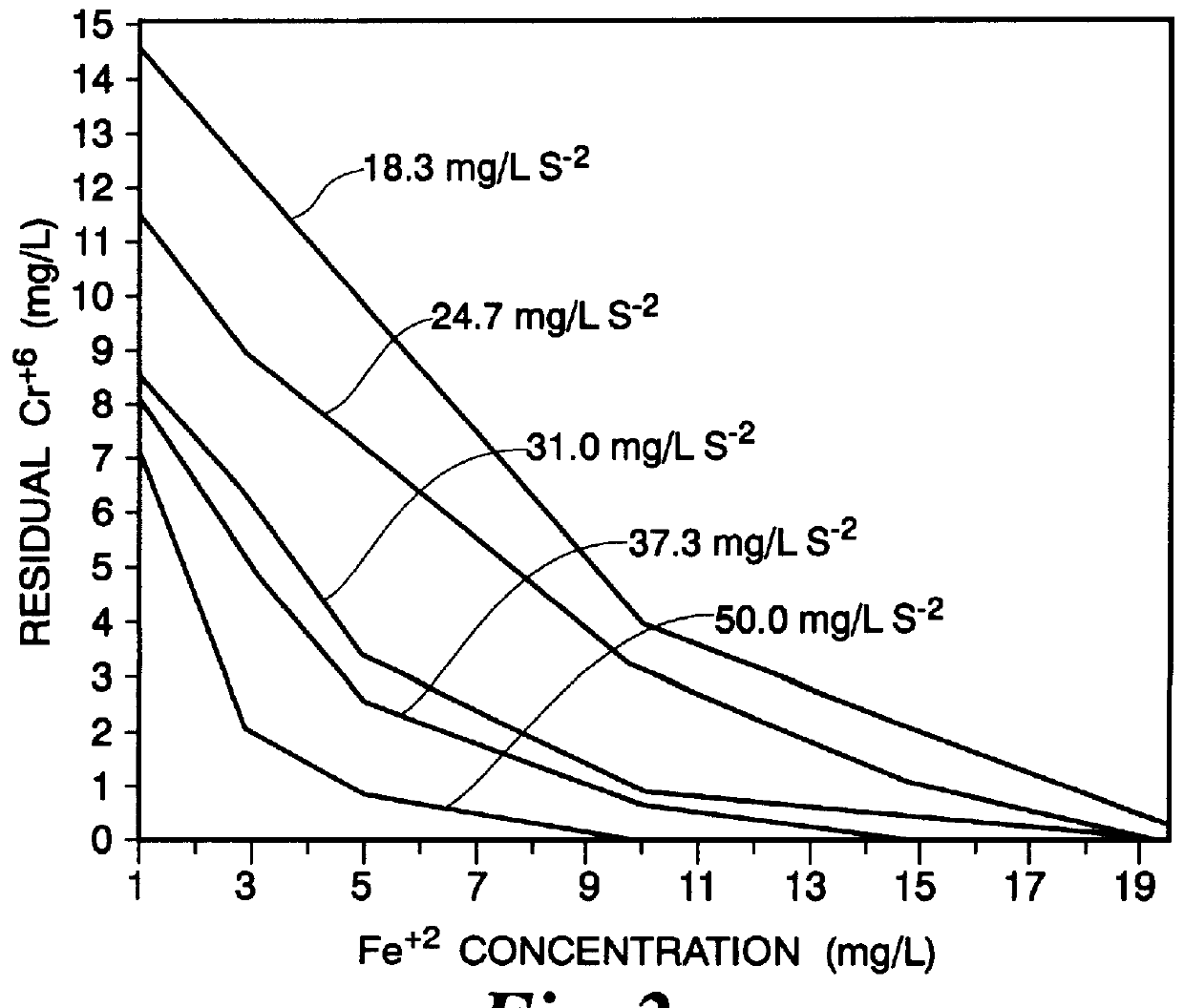
Analyses were made of Cr.sup.+6 reduction and heavy metal removal in distilled water and electroplating wastes. Jar tests were conducted using Phipps and Bird six-paddle stirrer with an illuminated base. Beakers were filled with 800 ml of distilled water and 10 ml of a 2,000 milligrams per liter (mg / L) Cr.sup.+6 solution. The pH of each solution was adjusted with either caustic or lime. Appropriate volumes of 1,000 mg / L S.sup.-2 solution (Na.sub.2 S / 9H.sub.2 O) was added to each beaker. The solutions were stirred at 100 rpm while the pH of each was adjusted as desired. After six minutes of stirring, the appropriate volume of a 1,000 mg / L ferrous solution (as FeSO.sub.4 / 7H.sub.2 O) was added. It may be noted here that in the practice of the invention described herein the ferrous ion may be added in the form either as sulfate or chloride. The volume was brought to 1,000 ml with distilled water and pH adjusted again while the solutions were stirred at 100 rpm. After six minutes, mixin...
example ii
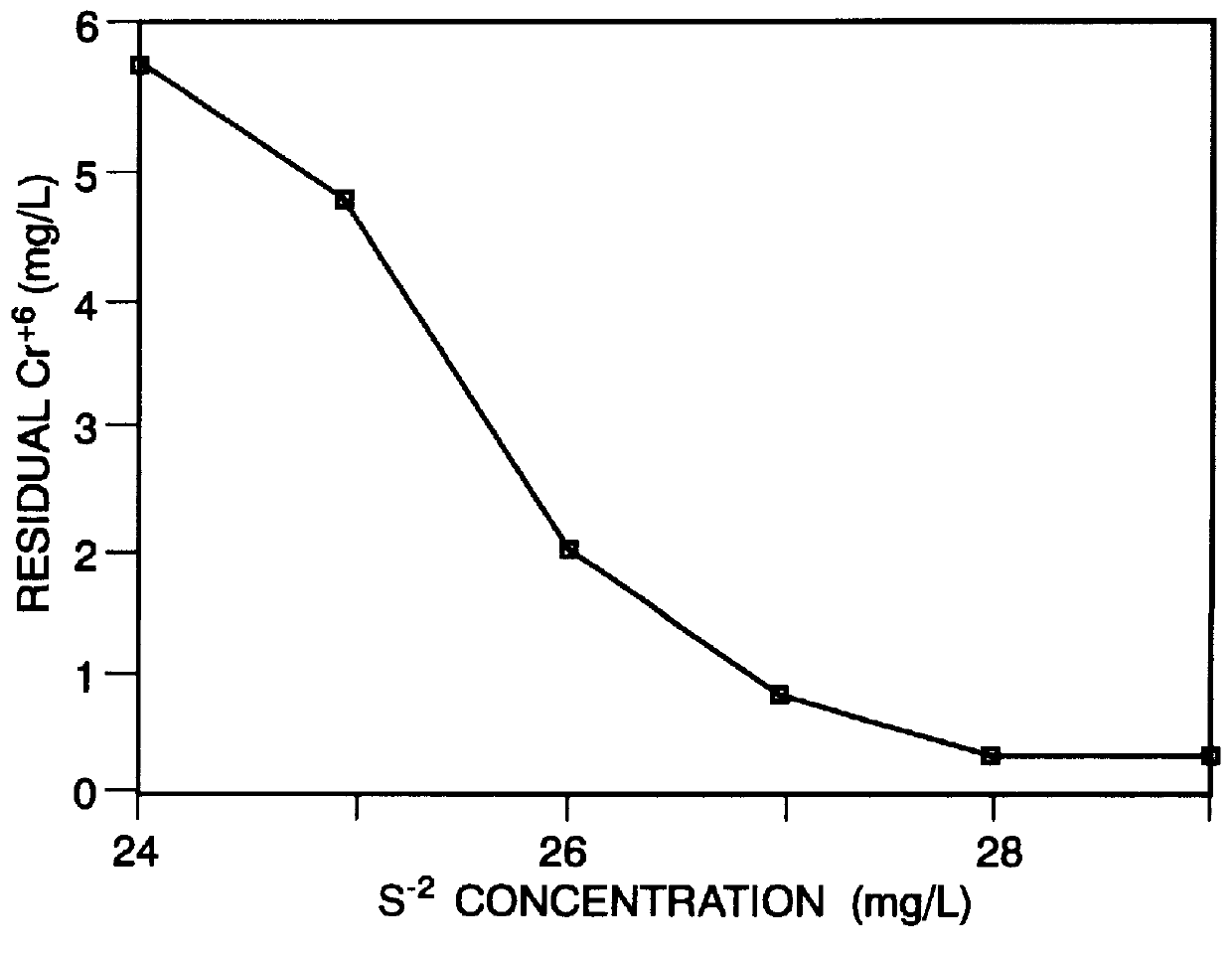
Jar tests on electroplating waste were conducted similarly to those of EXAMPLE I. Day-to-day concentration variations in the waste was ameliorated somewhat by collecting 40 gallons of electroplating waste from the subject ALC plant. Cr.sup.+6 concentration in the waste was extremely high (about 350 mg / L). Sufficient waste was diluted with distilled water to form a 100 liter solution of 55 mg / L Cr.sup.+6 for use in the tests in order to simulate average electroplating waste. One liter of the diluted waste solution was placed in a beaker and the pH adjusted to 7.2-7.5 with caustic (initial pH was 4.0). The solutions were stirred at 100 rpm while the desired volume of a 2,000 mg / L sulfide solution was added. Stirring continued for six minutes, at which time the appropriate volume of a 2,000 mg / L ferrous solution was added. The pH was adjusted and stirring continued for six minutes at 100 rpm. Stirring was slowed to 20 rpm for two minutes to allow floc formation; stirring was then stopp...
example iii
Waste water typically comprising influent to the subject ALC waste treatment plant was collected for jar testing. Analysis of the influent is shown in TABLE VI. The pH was lowered from 9.5 to 7.3 with nitric acid and beakers were filled with one liter of the resulting solution. Each solution was stirred at 100 rpm as the desired volume of 1,000 mg / L sulfide solution was added and stirring was continued for six minutes. The appropriate volume of 1,000 mg / L ferrous solution was added, pH adjusted, and stirring continued for six minutes at 100 rpm. Stirring was slowed to 20 rpm for two minutes to allow floc formation, pH adjustments were made, and stirring was stopped. The solutions sat undisturbed for two minutes, and if necessary were filtered through cotton.
TABLE VI ______________________________________ Metal Concentration Constituent (mg / L) ______________________________________ Cr.sup.+6 9.00 Cr 9.10 Fe 1.42 Cd 0.10 Cu 0.02 Ni 0.97 Pb 0.15 Zn 0.13 ________________________________...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Percent by volume | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap