Hydroxyindoles, their use as inhibitors of phosphodiesterase 4 and process for their preparation
a technology of phosphodiesterase and hydroxyindoles, which is applied in the field of new, substituted hydroxyindoles, can solve the problems of reducing the life expectancy of patients, affecting the quality of life of patients, and obvious respiratory distress of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
N-(3,5-Dichloropyridin-4-yl)-2-[1-(4-fluorobenzyl)-5-hydroxyindol-3-yl]-2-o xoacetamide
1.4 g of N-(3,5-dichloropyridin-4-yl)-2-[1-(4-fluorobenzyl)-5-methoxyindol-3-yl]-2- oxoacetamide (3 mmol) is dissolved in 100 ml of dichloromethane. The solution is heated to reflux and treated with a solution of 14 mmol of BBr.sub.3 in 15 ml of dichloromethane with stirring. The reaction mixture is refluxed for 3 hours. After cooling, the solution is intensively stirred for 3 hours at 20.degree. C. with 200 ml of an aqueous sodium hydrogencarbonate solution. The product crystallizes out, it is isolated, dried at 60.degree. C. and recrystallized from 80 ml of ethanol. Yield: 1.1 g (80% of theory) Melting point: 213-214.degree. C.
example 2
N-(3,5-Dichloropyridin-4-yl)-2-[1-(4-fluorobenzyl)-5-hydroxyindol-3-yl)-]2- oxoacetamide
5 g (38 mmol) anhydrous aluminium chloride is introduced into 50 ml ethane-1,2,-dithiol. A solution of 4.7 g of N-(3,5-dichloropyridin-4-yl)-2-[1-(4-fluorobenzyl)-5-methoxyindol-3-yl]-2- oxoacetamide (10 mmol) in 50 ml of dichloromethane is added at 0.degree. C. The mixture is stirred at 0.degree. C. for 4 hours. 50 ml of 10% hydrochloric acid is added dropwise at from 0 to 10.degree. C. with stirring. The crystallizing product is isolated, washed with water and dried at 20.degree. C. A pure product is obtained by recrystallization from ethanol (180 ml). Yield: 3.1 g (67% of theory) Melting point: 212-214.degree. C.
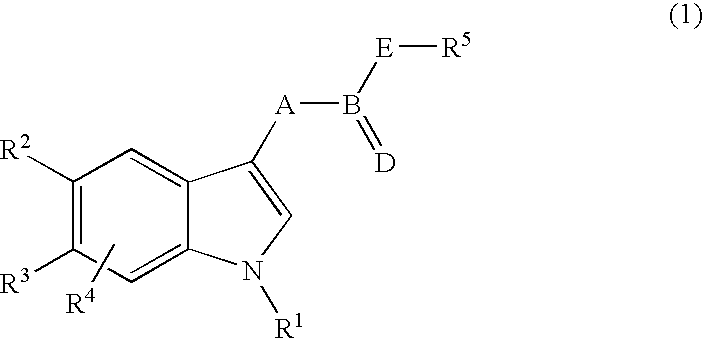
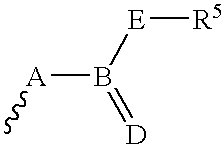
Exemplary preparative process as follows for compounds of Formula (1) from starting substances of the type described, in which R.sup.7 is an acyl, alkoxycarbonyl, aryloxycarbonyl, aminocarbonyl, N-substituted aminocarbonyl, silyl or sulfonyl residue.
example 3
N-(3,5-Dichloropyridin-4-yl)-2-[1-(4-fluorobenzyl)-5-hydroxyindol-3-yl]-2-o xoacetamide Na salt
5 g of N-(3,5-dichloropyridin-4-yl)-2-[5-acetoxy-1-(4-fluorobenzyl)-indol-3-yl]2- oxoacetamide (10 mmol) are stirred at 40.degree. C.-50.degree. C. for 1 hour in 50 ml dilute sodium hydroxide solution. The solution is neutralized with 10% hydrochloric acid while cooling with ice, and is concentrated to dryness. The residue is dissolved in 80 mle acetone and insoluble constituents are removed. The clear solution is treated with a solution of 0.4 g NaOH in 3 ml of water and stirred at 20.degree. C. for 2 hours. The crystallized product is isolated, washed with acetone and dried at 60.degree. C. Yield: 2.44 g (51% of theory) Melting point: 265.degree. C.
An exemplary preparation process follows for compounds of Formula (1) from other compounds of Formula (1).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com