Electrochemical biosensor test strip
a biosensor and electrochemical technology, applied in the field of biosensors, can solve the problems of large test strips, large error chances of test results, and large size of test strips, and achieve the effects of accurate test results, reduced error chances, and good color contras
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
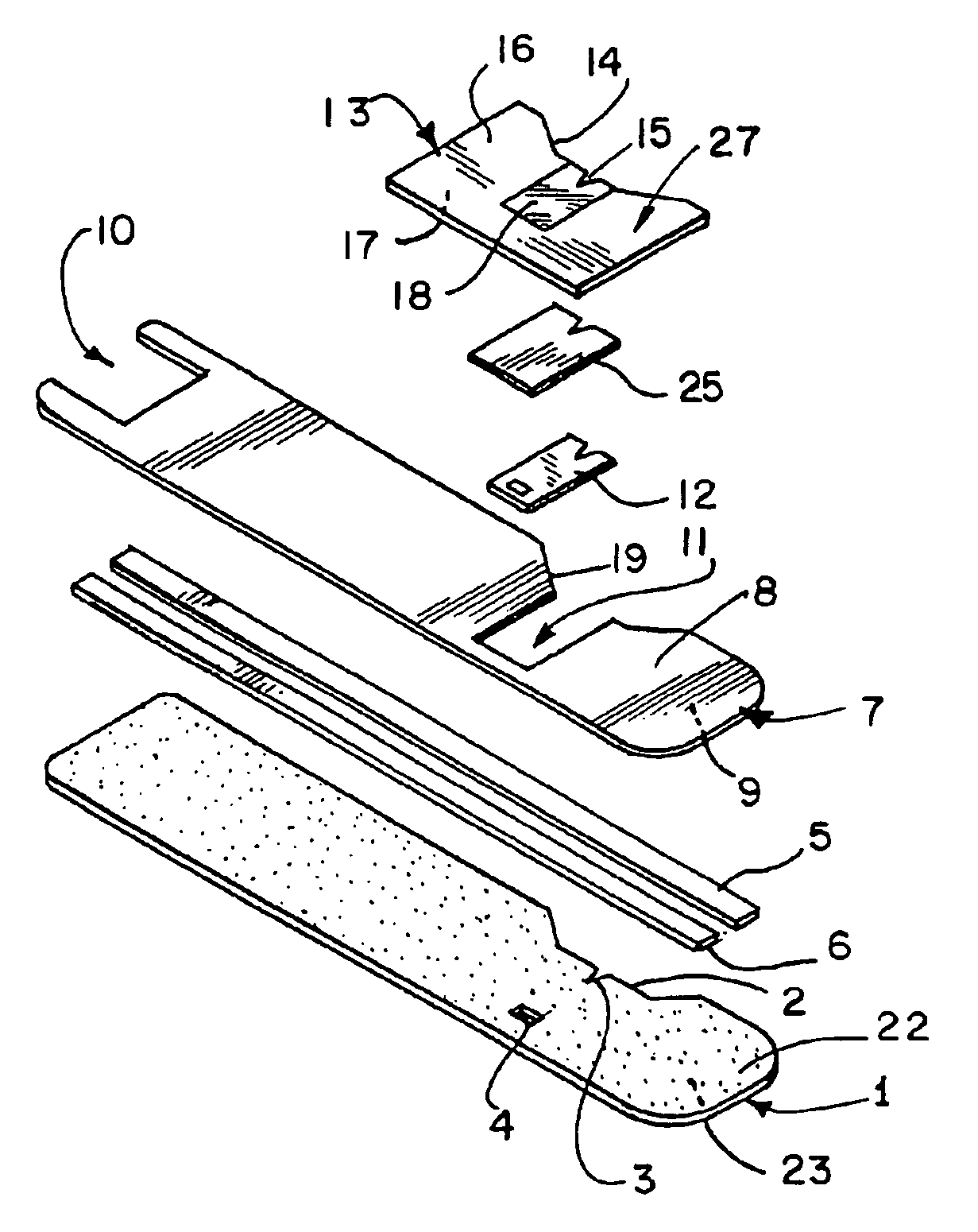
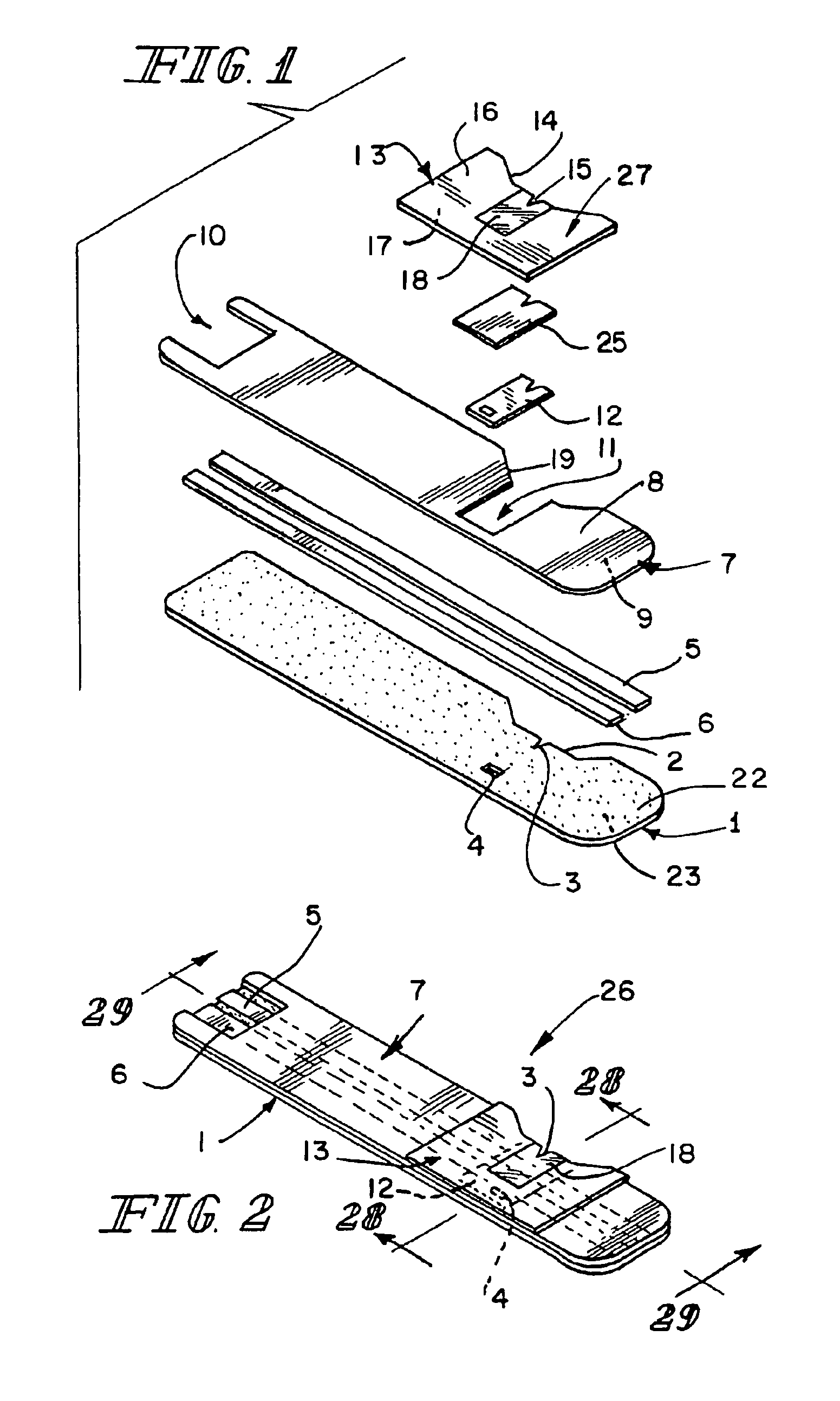
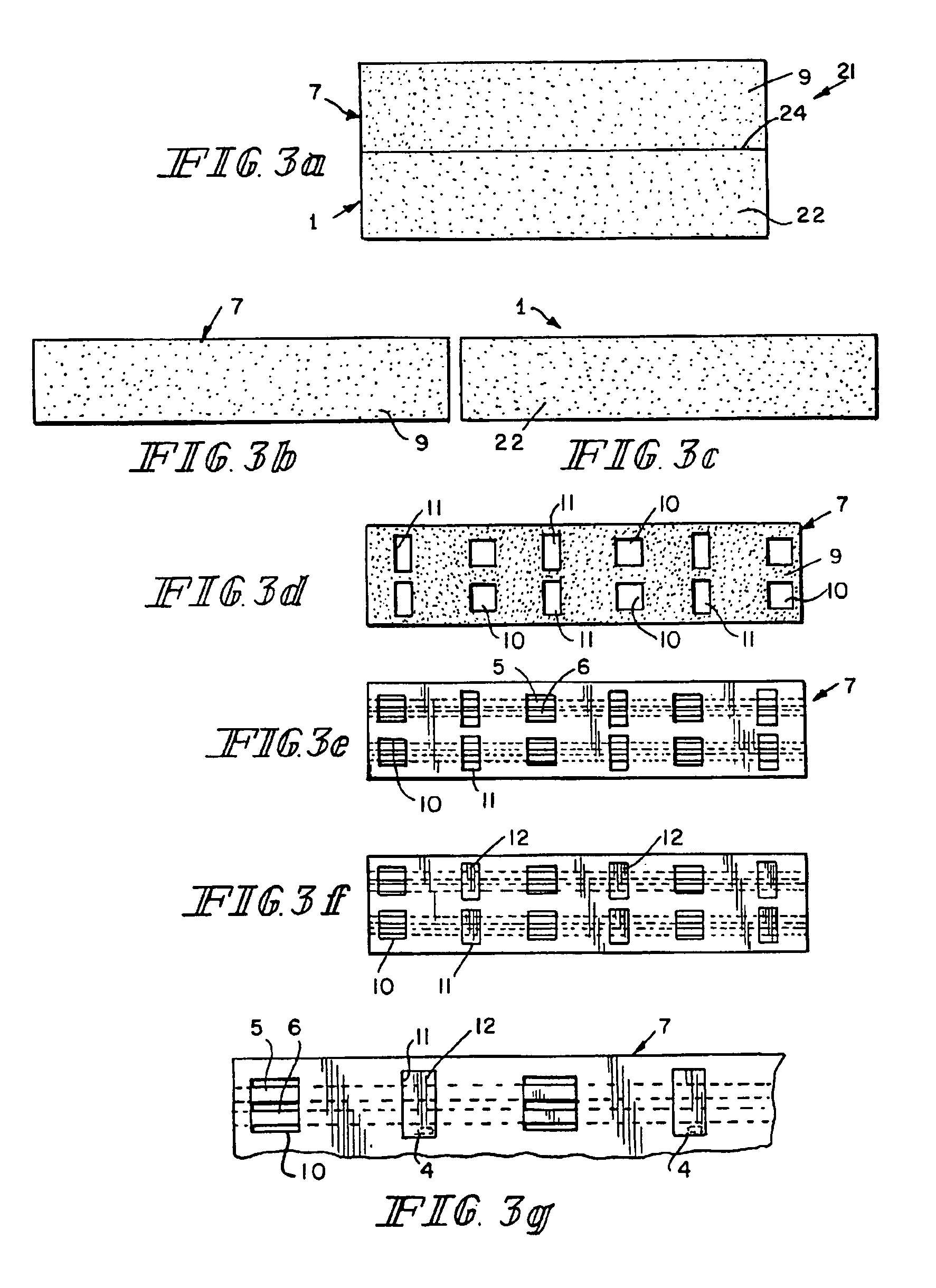
[0022]The components of a preferred embodiment of the present inventive biosensor are shown in FIGS. 1, 2, 4 and 5. The biosensor includes first insulating substrate 1, which has first surface 22 and second surface 23. Insulating substrate 1 may be made of any useful insulating material. Typically, plastics, such as vinyl polymers, polyimides, polyesters, and styrenics provide the electrical and structural properties which are desired. First insulating substrate 1 further includes indentation 2, notch 3, and vent hole 4. Because the biosensor shown in FIG. 1 is intended to be mass produced from rolls of material, necessitating the selection of a material which is sufficiently flexible for roll processing and at the same time sufficiently stiff to give a useful stiffness to the finished biosensor, a particularly preferred first insulating substrate 1 is 7 mil thick MELINEX 329 plastic, a polyester available from ICI Films (3411 Silverside Road, PO Box 15391, Wilmington, Del. 19850).
[...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mean molecular weight | aaaaa | aaaaa |
| mean molecular weight | aaaaa | aaaaa |
| transparent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com