Improved method for preparing N-methyl-3-phenyl-3-hydroxy-propylamine
A technology of phenyl and hydroxyl, which is applied in the field of preparation of N-methyl-3-phenyl-3-hydroxy-propylamine, which can solve the problems of high cost, low yield and difficult industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
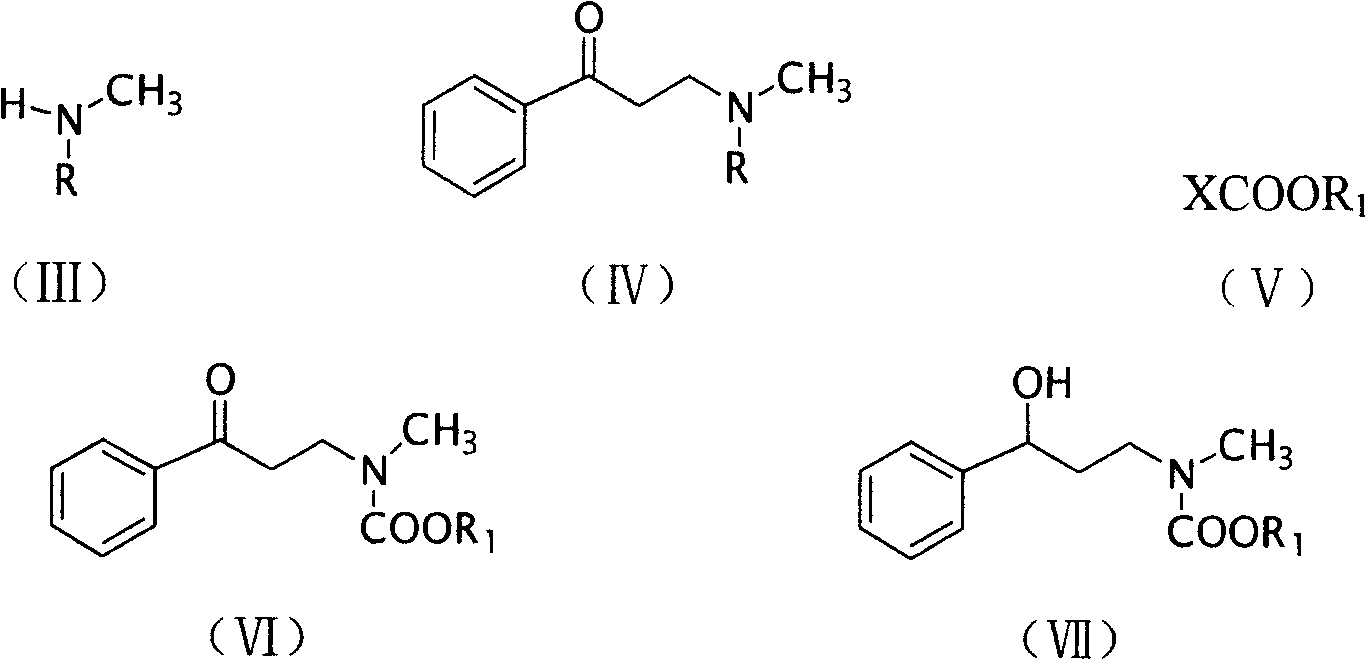
[0016] The method for preparing N-methyl-3-phenyl-3-hydroxyl-propylamine of the present invention is characterized in that said preparation method comprises the steps:
[0017] (1) Compound (II), paraformaldehyde and compound (III) are placed in C 1 ~C 4 In the monohydric alcohol, the molar ratio of compound (II), paraformaldehyde and compound (III) is 1: (1.0~4.0): (1.0~3.0), carry out Mannich reaction under the condition that has acid catalyst presence to obtain compound (IV );
[0018] Among them, the recommended acid catalysts are HCl, H 2 SO 4 , PCl 3 , PCl 5 or POCl 3 , the preferred monohydric alcohol is methanol, ethanol, propanol or butanol.
[0019] (2) Place compound (IV) and compound (V) in an aprotic solvent, and in the presence of a base, reflux for 1 to 3 hours to obtain compound (VI);
[0020] Among them, the recommended aprotic solvent is benzene, toluene or xylene, and the recommended base is KOH, NaOH, Ba(OH) 2 , Ca(OH) 2 , Na 2 CO 3 , K 2 CO 3...
Embodiment 1
[0028] (1) N-methyl-N-benzyl-3-phenyl-3-carbonyl-propylamine hydrochloride:
[0029] Add 84.7g (0.72mol) of N-methylbenzylamine and 250mL of absolute ethanol into a 500mL three-necked flask, and feed HCl gas into the ice-water bath until the solution pH<3, then add 26.2g (0.87mol) of paraformaldehyde and 70g (0.58mol) of acetophenone was heated, stirred and refluxed for 7h, cooled naturally, stirred at room temperature for 3h, filtered with suction, the filter cake was washed with ethanol, and dried to obtain 154.4g of white solid, yield: 91.5%. mp 192~194°C, HPLC content 99.9%.
[0030] 1 H-NMR(DMSO)δ: 2.70(s, 3H, N-CH 3 ), 3.20~3.50 (m, 2H, N-CH 2 ), 3.68(t, 2H, CO-CH 2 , J=7.5Hz), 4.35 (AB, 2H, Ar-CH 2 , J=13.0Hz), 7.40~8.00 (m, 10H, Ar-H).
[0031] (2) N-methyl-N-ethoxycarbonyl-3-phenyl-3-carbonyl-propylamine:
[0032] With 116g (0.40mol) N-methyl-N-benzyl-3-phenyl-3-carbonyl-propylamine hydrochloride, 42.4g (0.40mol) Na 2 CO 3 and 52.1g (0.48mol) ClCO 2 Add Et i...
Embodiment 2
[0041] (1) N-methyl-N-benzyl-3-phenyl-3-carbonyl-propylamine hydrochloride:
[0042] Add 84.7g (0.72mol) of N-methylbenzylamine and 250mL of absolute ethanol into a 500mL three-necked flask, add 75mL of concentrated hydrochloric acid dropwise in an ice-water bath, and add 26.2g (0.87mol) of paraformaldehyde and 70g (0.58mol) of acetophenone was heated, stirred and refluxed for 7h, cooled naturally, stirred at room temperature for 3h, filtered with suction, the filter cake was washed with ethanol, and dried to obtain 154.4g of white solid, yield: 91.5%. mp 192-194°C.
[0043] (2) N-methyl-N-phenoxycarbonyl-3-phenyl-3-carbonyl-propylamine:
[0044] 116g (0.40mol) N-methyl-N-benzyl-3-phenyl-3-carbonyl-propylamine hydrochloride, 42.4g (0.40mol) Na 2 CO 3 and 74.8g (0.48mol) ClCO 2 Ph was added to a 1000mL three-necked flask, and 300mL toluene was added as a solvent, heated to reflux for 3.5h, cooled, suction filtered, the filter cake was washed with a small amount of toluene, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com