Use of phenoxyacetic acid derivatives for treating hyperactive bladder
A drug, bladder technology, applied in the field of new indications, can solve problems such as the treatment effect of overactive bladder that has not been mentioned
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
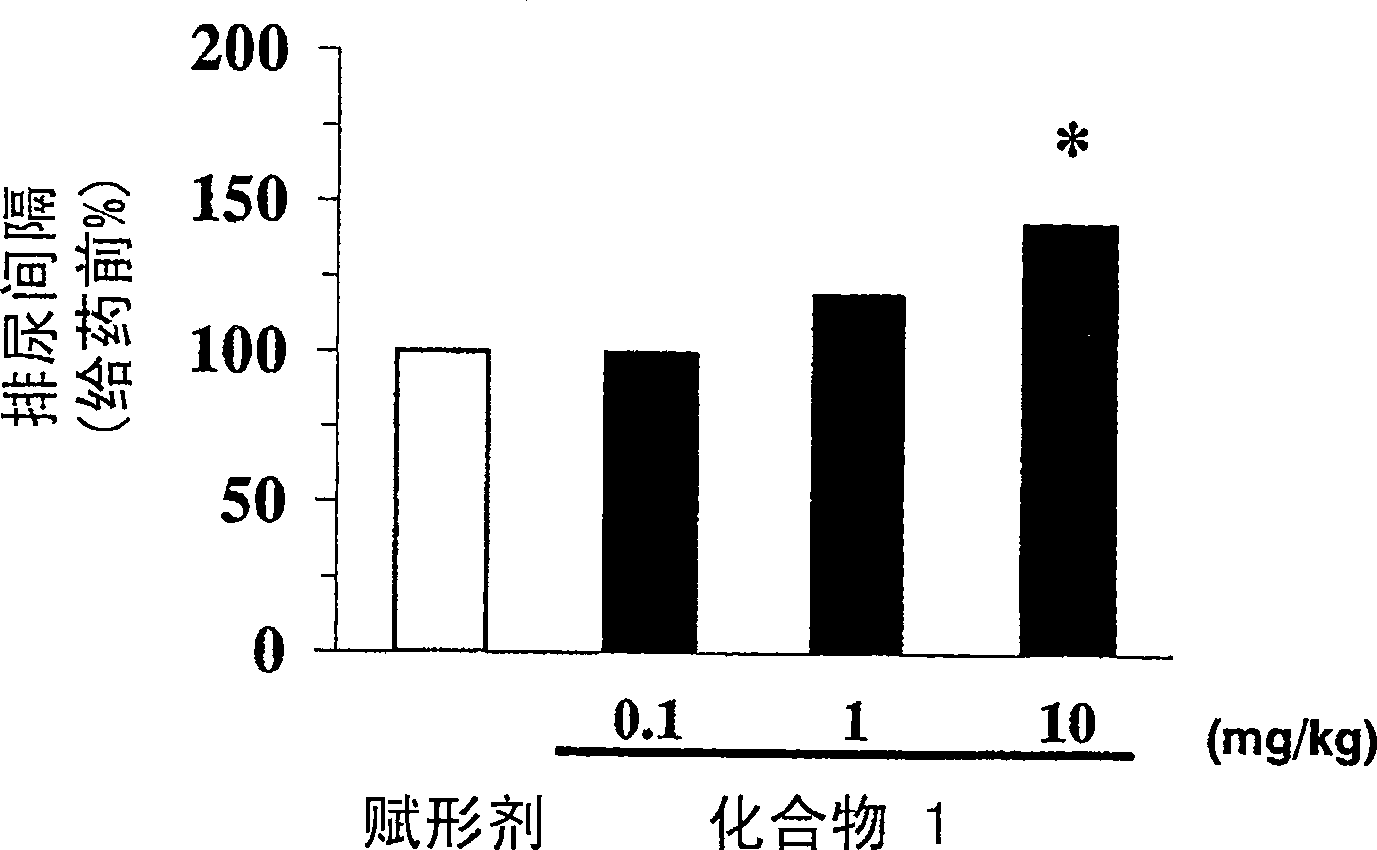
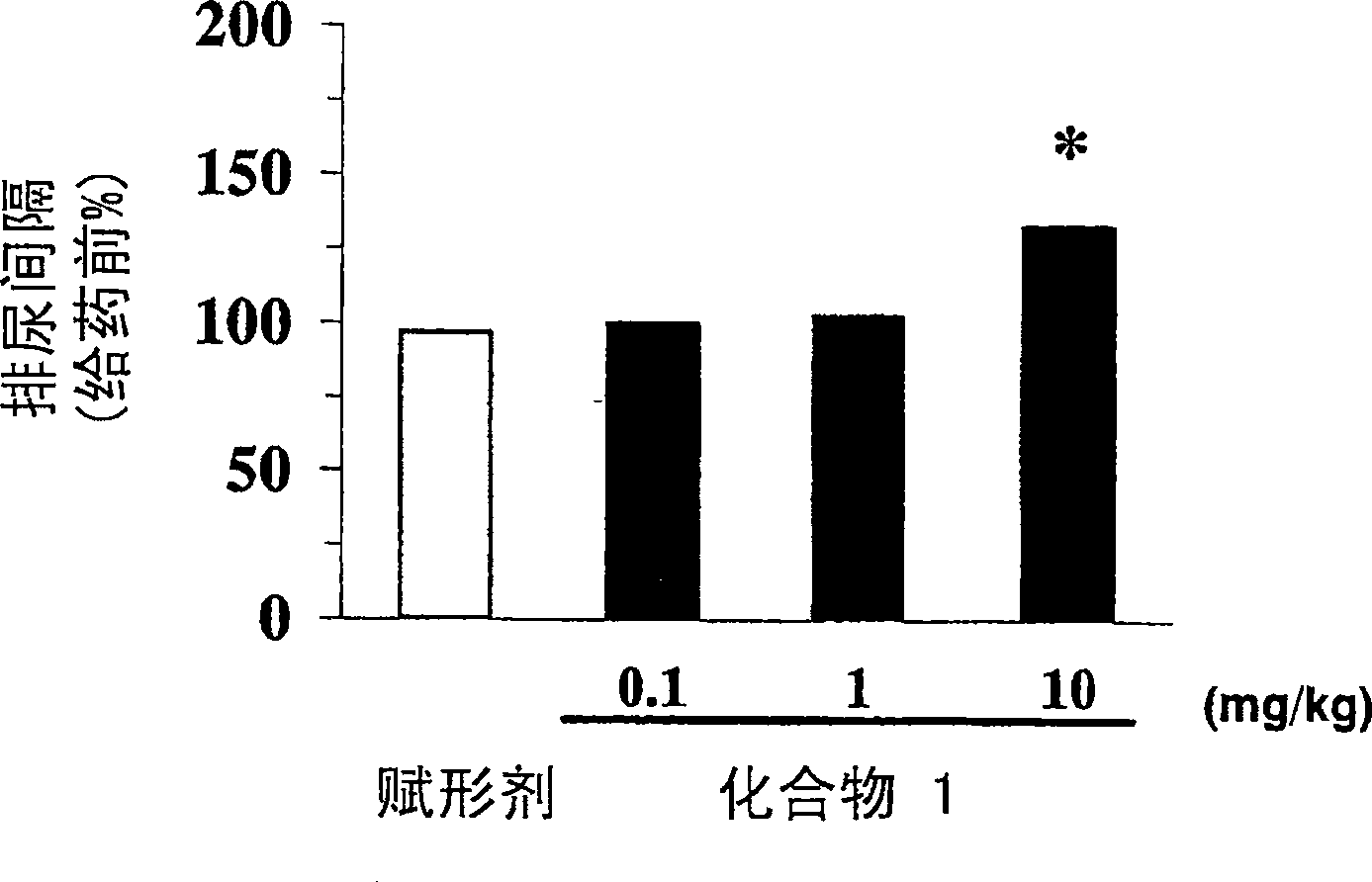
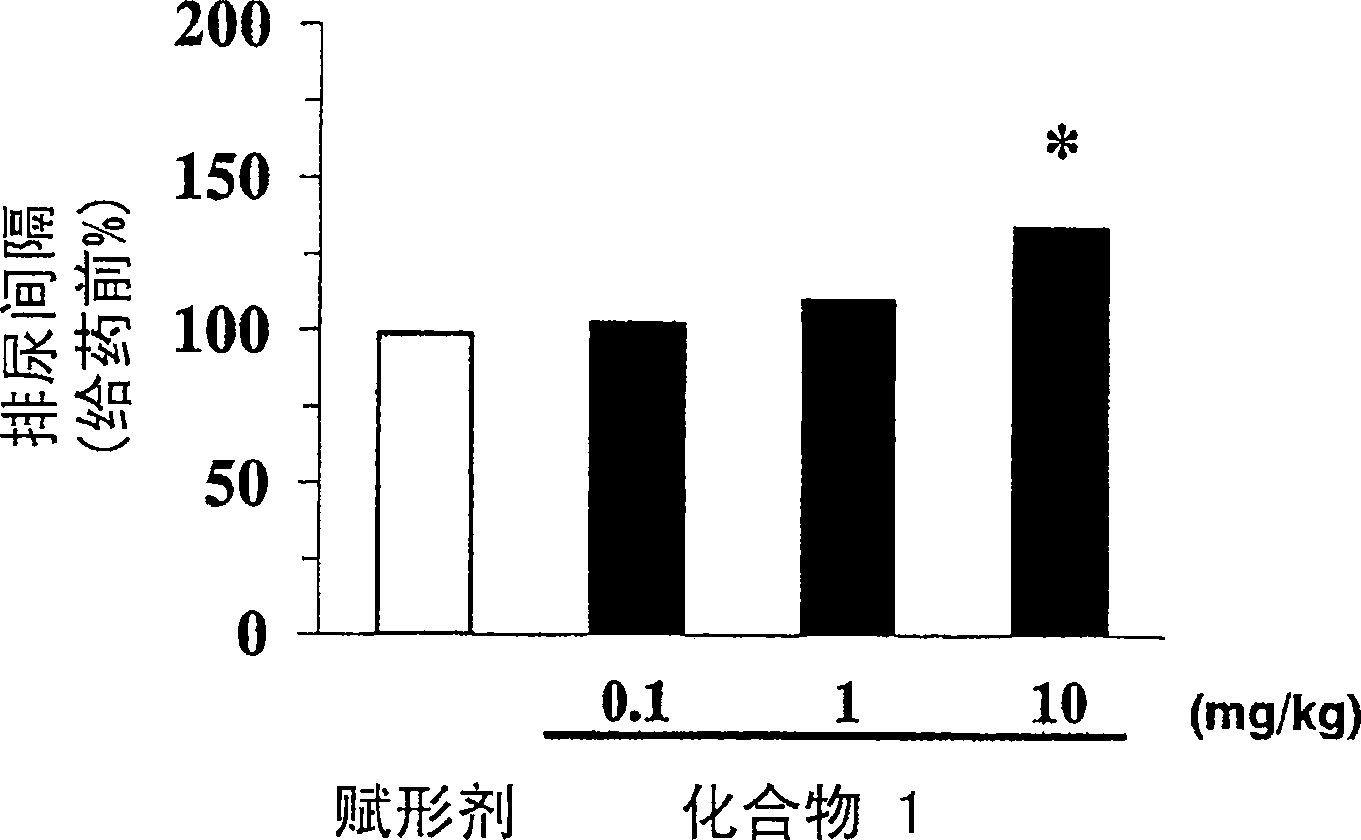
[0013] The present invention relates to the use of phenoxyacetic acid derivatives as described in EP1095932 in the preparation of medicines for treating overactive bladder and pharmaceutical preparations for treating overactive bladder, the medicines or pharmaceutical preparations comprising The above-mentioned phenoxyacetic acid derivatives are used as active ingredients. According to EP1095932, the compounds forming the basis of the use and formulations according to the invention are beta-3-adrenoceptor agonists. In particular, these substances are useful in the treatment of neurogenic hyperbladder, neurogenic detrusor hyperactivity and in the treatment of congenital hyperbladder and congenital detrusor hyperactivity.
[0014] The compounds forming the basis of the applications and formulations of the present invention are represented by the following general formula I:
[0015] Chemical formula I:
[0016]
[0017] in
[0018] X is a chiral carbon atom of R or S, pref...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com