Oxime ester compound and photopolymerization initiator comprising the compound
A technology of photopolymerization initiators and compounds, applied in the fields of optics, organic chemistry, optomechanical equipment, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
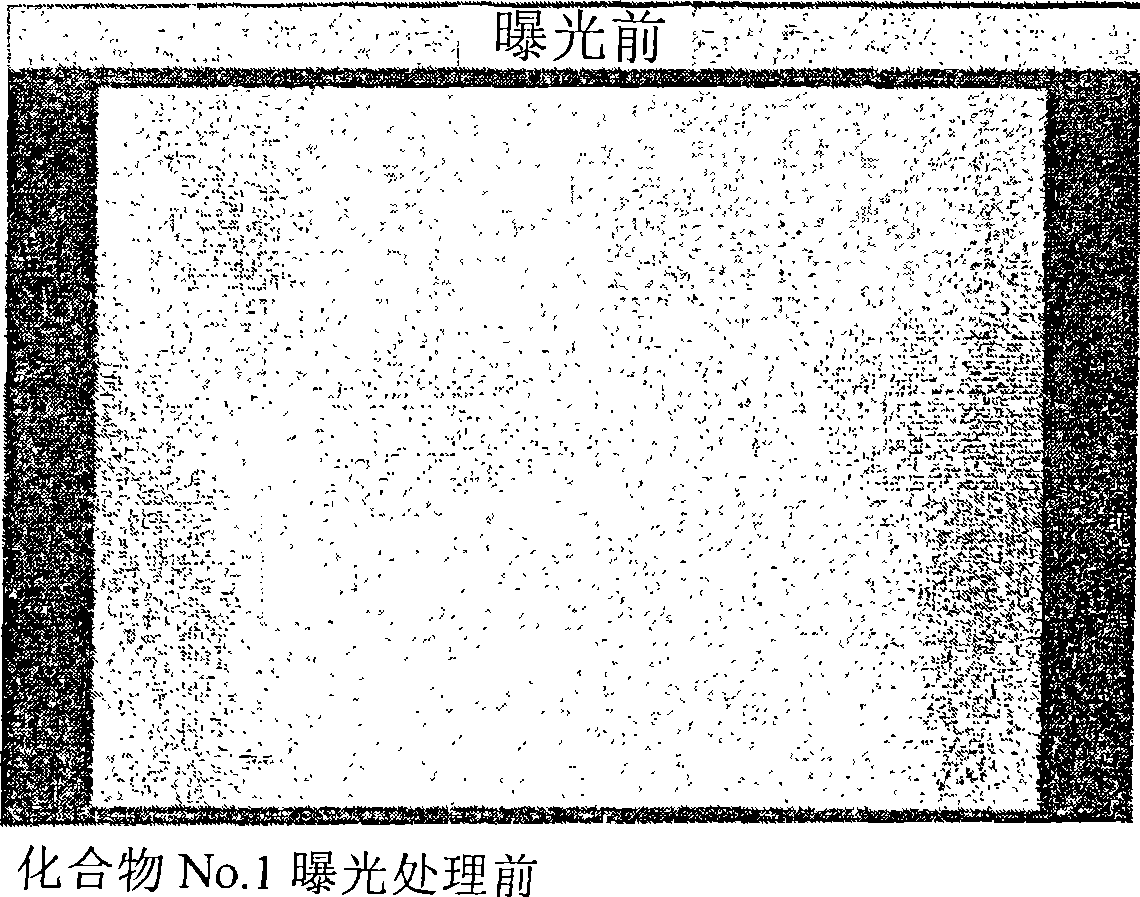
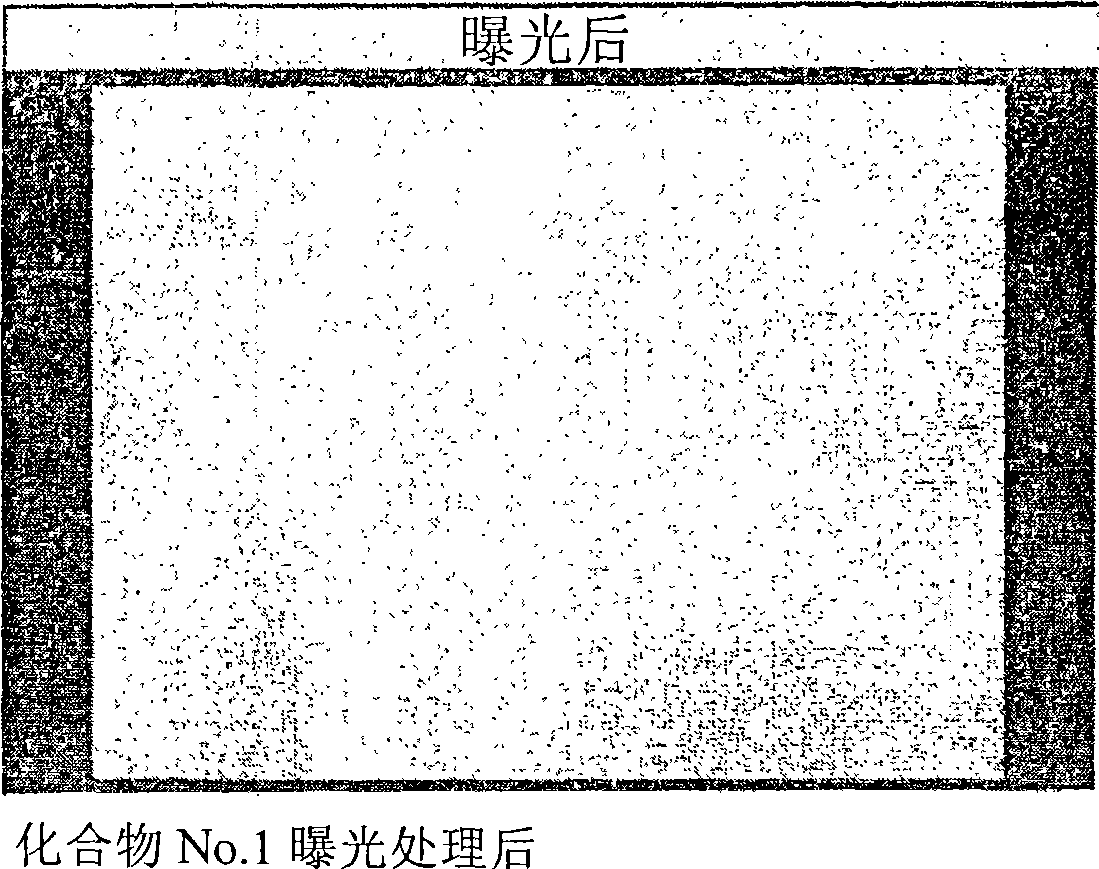
[0051] [Example 1] Preparation of Compound No.1
[0052] Production of 4-fluoro-2-methylbenzoic acid chloride
[0053] 4-Fluoro-2-methylbenzoic acid chloride having the structure represented by the following chemical formula was prepared as follows.
[0054] [chemical formula 6]
[0055]
[0056] Add 20.0g (0.13 mole) 4-fluoro-2-methylbenzoic acid, 0.10g (1.3 mmol) N, N-dimethylformamide and 134g toluene under nitrogen atmosphere, add dropwise 23.2g ( 0.20 mol) of thionyl chloride, heated to 60-65°C and stirred for 1 hour. The solvent was distilled off and dried at 50° C. under reduced pressure to obtain 19.7 g (yield 88%, GC purity 99%) of a brown liquid.
[0057] The IR measurement results of the obtained brown liquid are shown below, and it was confirmed that the brown liquid was the target object.
[0058] IR measurement: (cm -1 )
[0059] 2985, 2931, 1766, 1606, 1579, 1486, 1450, 1384, 1240, 1189, 1105, 964, 869, 823
[0060] Production of acyl body
[0061] ...
Embodiment 2
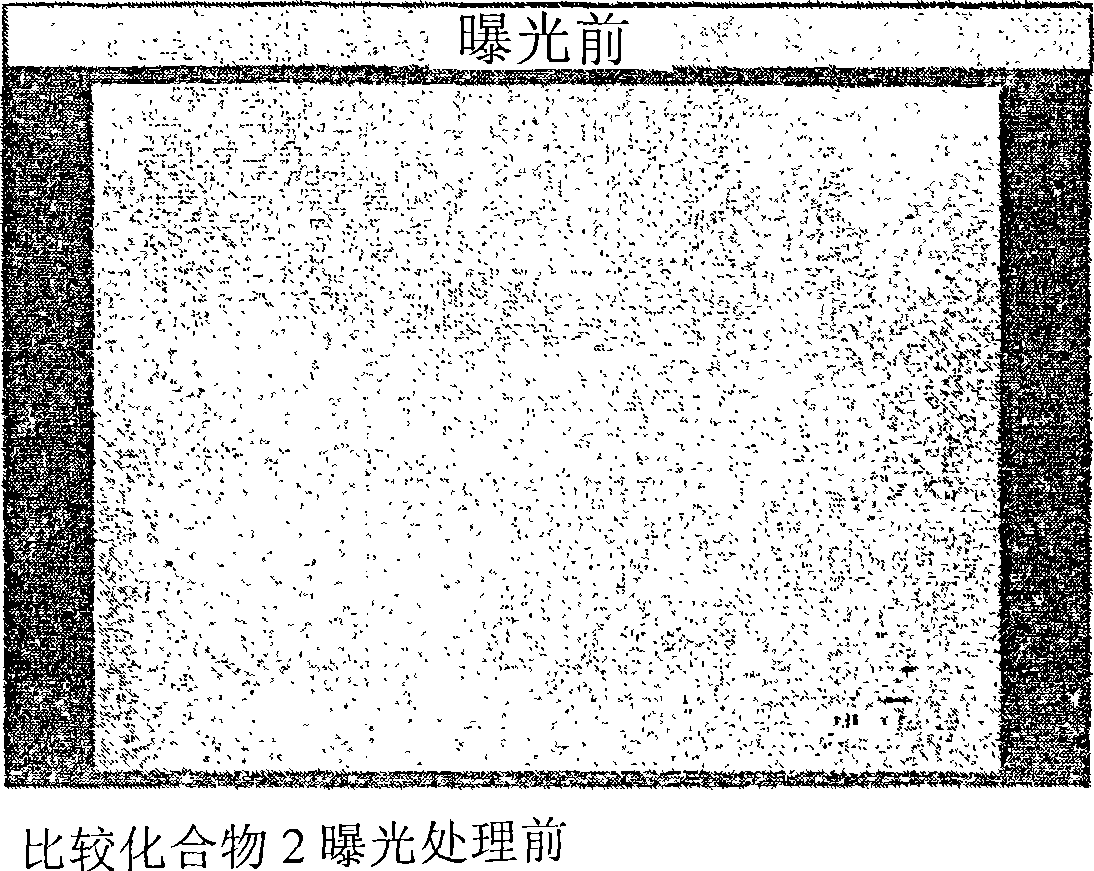
[0083] [Example 2] Production of Photosensitive Composition No.1
[0084] With respect to 14g of acrylic copolymer, add 5.9g of trimethylolpropane triacrylate, 2.7g of compound No.1 and 79g of ethyl cellosolve obtained in Example 1, and stir well to obtain a photosensitive composition No.1.
[0085] In addition, the above-mentioned acrylic copolymer is obtained through the following steps: 20 parts by mass of methacrylic acid, 15 parts by mass of hydroxyethyl methacrylate, 10 parts by mass of methyl methacrylate and 55 parts by mass of methyl Butyl acrylate was dissolved in 300 parts by mass of ethyl cellosolve, and 0.75 parts by mass of azobisisobutyronitrile was added under a nitrogen atmosphere, and reacted at 70°C for 5 hours to obtain the above-mentioned acrylic copolymer .
Embodiment 3
[0086] [Example 3] Production of photosensitive composition No.2
[0087] With respect to 7.2g of acrylic copolymer, add 4.3g of trimethylolpropane triacrylate, 2.0g of compound No.1 obtained in Example 1 and 87g of ethyl cellosolve, and fully stir to obtain a photosensitive combination Object No.2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com