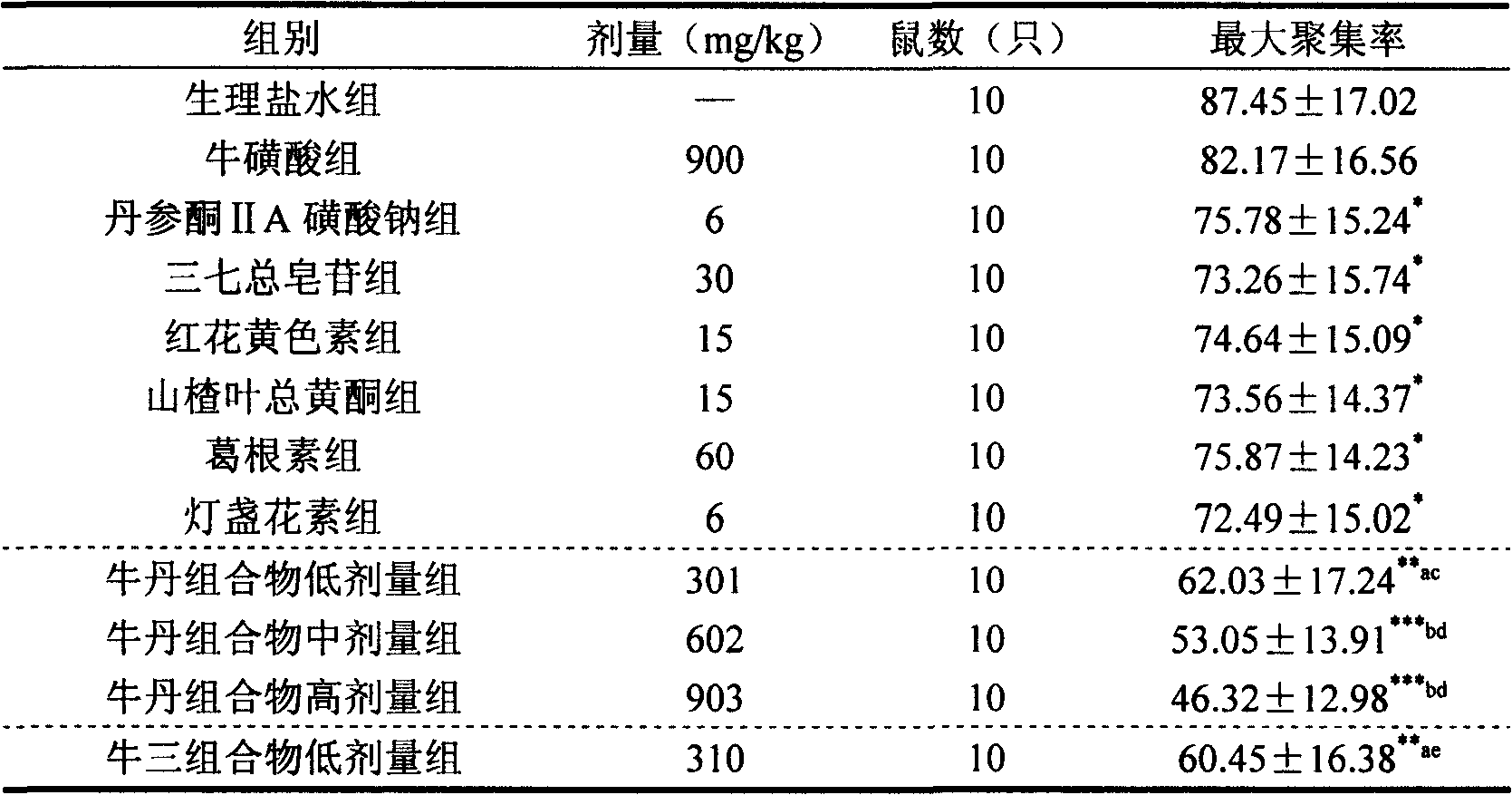
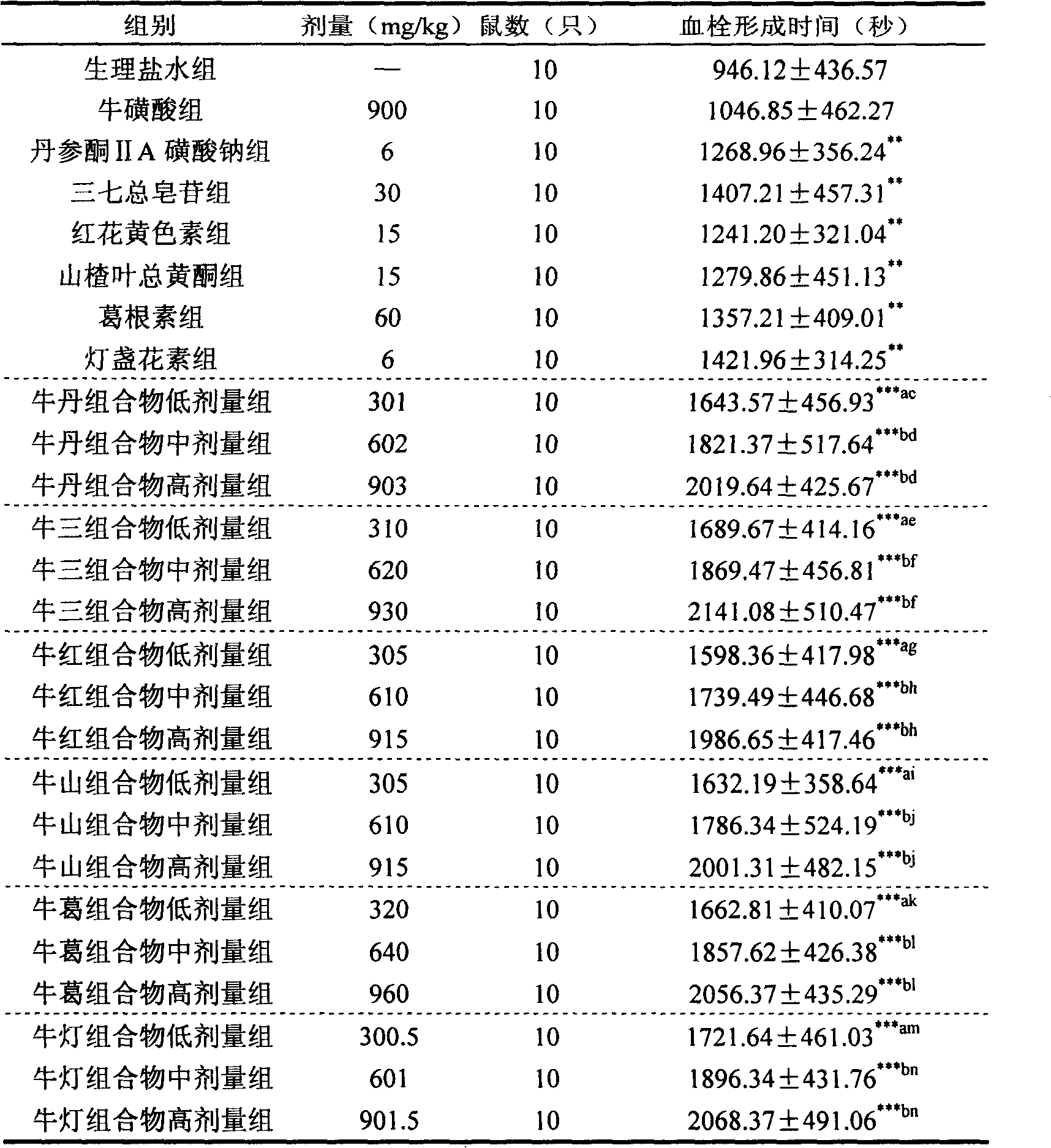
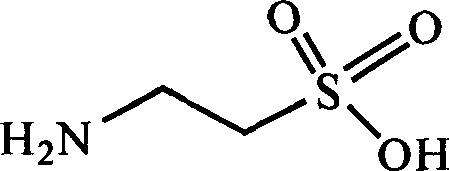
Taurine and medical combination for treating cardiovascular and cerebrovascular diseases
A technology for cardiovascular and cerebrovascular diseases and compositions, applied in the field of medicine, to prolong the time of carotid artery thrombosis, improve cerebral circulation, and reduce cerebral ischemia-reperfusion injury
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0193] Embodiment 1 Preparation of the pharmaceutical composition aqueous injection of the present invention
[0194] prescription:
[0195] Prescription 1: Niudan Injection
[0196] Taurine 3kg
[0197] Sodium Tanshinone IIA Sulfonate 10g
[0198] Polysorbate 80 40g
[0199] PEG400 300ml
[0200] Add water for injection to 10000ml
[0201] A total of 1000 sticks were prepared
[0202] Prescription 2: Niusan Injection
[0203] Taurine 3kg
[0204] Panax notoginseng saponins 100g
[0205] Polysorbate 80 40g
[0206] PEG400 300ml
[0207] Add water for injection to 10000ml
[0208] A total of 1000 sticks were prepared
[0209] Prescription 3: Niuhong Injection
[0210] Taurine 3kg
[0211] Safflower Yellow 50g
[0212] Polysorbate 80 40g
[0213] PEG400 200ml
[0214] Add water for injection to 10000ml
[0215]A total of 1000 sticks were prepared
[0216] Prescription 4: Niushan Injection
[0217] Taurine 3kg
[0218] Hawthorn Leaf Total Flavonoids 50g
...
Embodiment 2
[0249] Embodiment 2 Preparation of the powder injection of the pharmaceutical composition of the present invention
[0250] prescription:
[0251] Prescription 1: Niudan for injection
[0252] Taurine 3kg
[0253] Sodium Tanshinone IIA Sulfonate 10g
[0254] Polysorbate 80 40g
[0255] PEG400 300ml
[0256] Mannitol 800g
[0257] Add water for injection to 10000ml
[0258] A total of 1000 sticks were prepared
[0259] Prescription 2: Niusan for injection
[0260] Taurine 3kg
[0261] Panax notoginseng saponins 100g
[0262] Polysorbate 80 50g
[0263] PEG400 400ml
[0264] Mannitol 800g
[0265] Add water for injection to 10000ml
[0266] A total of 1000 sticks were prepared
[0267] Prescription 3: Bull Red for Injection
[0268] Taurine 3kg
[0269] Safflower Yellow 50g
[0270] Polysorbate 80 30g
[0271] PEG400 200ml
[0272] Mannitol 800g
[0273] Add water for injection to 10000ml
[0274] A total of 1000 sticks were prepared
[0275] Prescription...
Embodiment 3
[0310] Embodiment 3 Preparation of the pharmaceutical composition sodium chloride infusion of the present invention
[0311] prescription:
[0312] Prescription 1: Niudan Sodium Chloride Injection
[0313] Taurine 3kg
[0314] Sodium Tanshinone IIA Sulfonate 10g
[0315] Polysorbate 80 50g
[0316] PEG400 400ml
[0317] Sodium chloride 4500g
[0318] Add water for injection to 500000ml
[0319] A total of 1000 bottles were prepared
[0320] Prescription 2: Bovine Sodium Trichloride Injection
[0321] Taurine 3kg
[0322] Panax notoginseng saponins 100g
[0323] Polysorbate 80 50g
[0324] PEG400 400ml
[0325] Sodium chloride 4500g
[0326] Add water for injection to 500000ml
[0327] A total of 1000 bottles were prepared
[0328] Prescription 3: Niuhong Sodium Chloride Injection
[0329] Taurine 3kg
[0330] Safflower Yellow 50g
[0331] Polysorbate 80 50g
[0332] PEG400 400ml
[0333] Sodium chloride 4500g
[0334] Add water for injection to 500000ml ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com