Nitride red phosphor and process for producing the same
一种红色荧光、荧光材料的技术,应用在红色氮化物荧光材料领域,能够解决荧光材料数量少等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0114] In order to obtain Eu powder as a raw material, metal europium is directly nitrided. In a nitrogen box, metallic europium (manufactured by Soekawa Chemical Co., Ltd., 99.9%, block) was filed with a file to obtain fine-grained metallic europium of 350 μm or less. The obtained fine-grained metal europium was charged into a carbon crucible and the crucible was kept at 500° C. for 15 minutes in a nitrogen atmosphere, then kept at 750° C. for 2 hours, further kept at 900° C. for 1 hour, and then in a furnace cool down. After cooling, the crucible was placed in a nitrogen box and the EuN was removed in a nitrogen atmosphere. This powder was pulverized into a powder of 150 μm or less and used as a raw material.
[0115] Subsequently, in order to obtain Ca 3 N 2 Powder, directly nitrided calcium metal. In a nitrogen box, metallic calcium (manufactured by Wako Pure Chemical Co., Ltd., 99.0%, granular) was charged into a carbon crucible and the crucible was kept at 450° C. f...
Embodiment 2
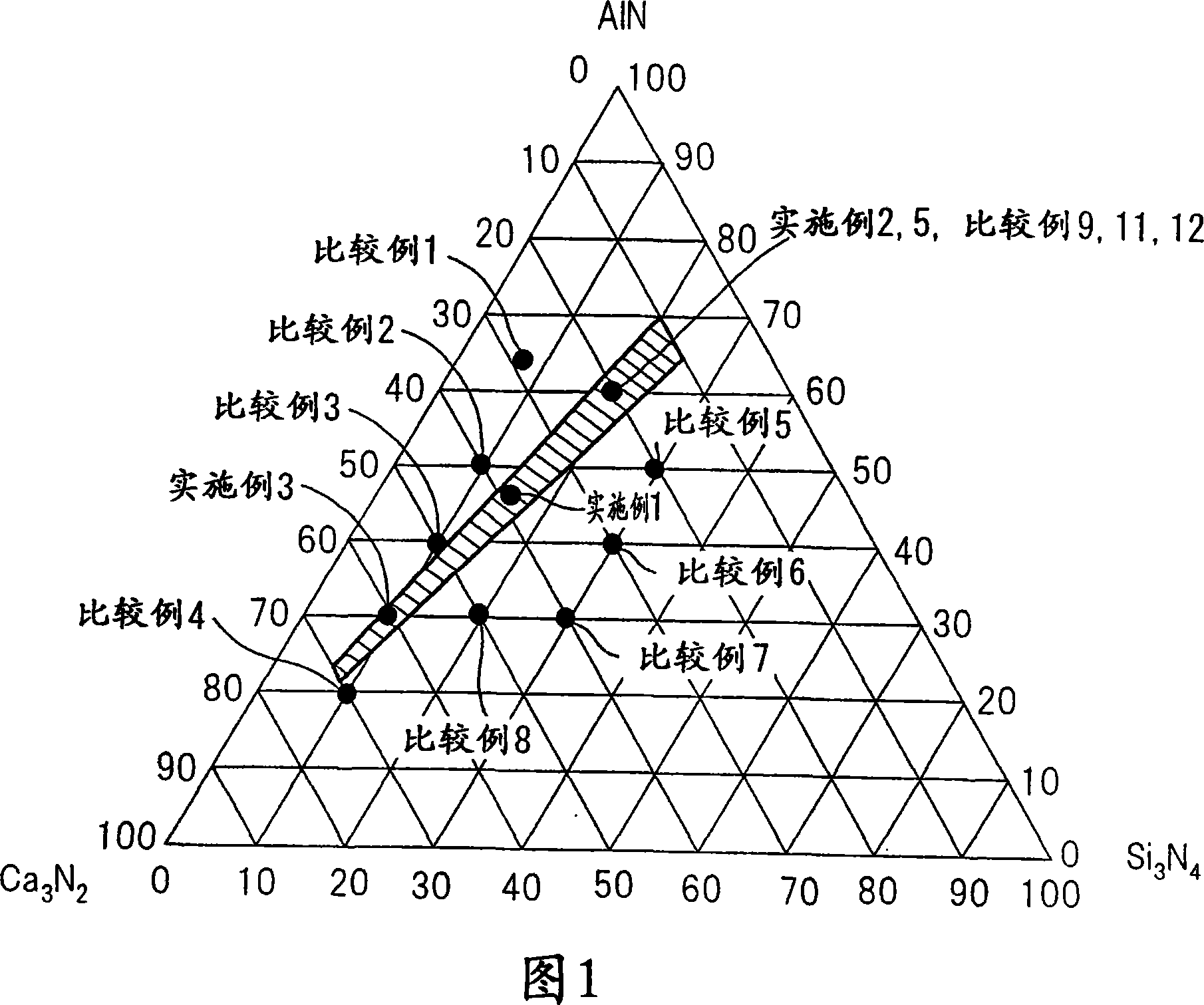
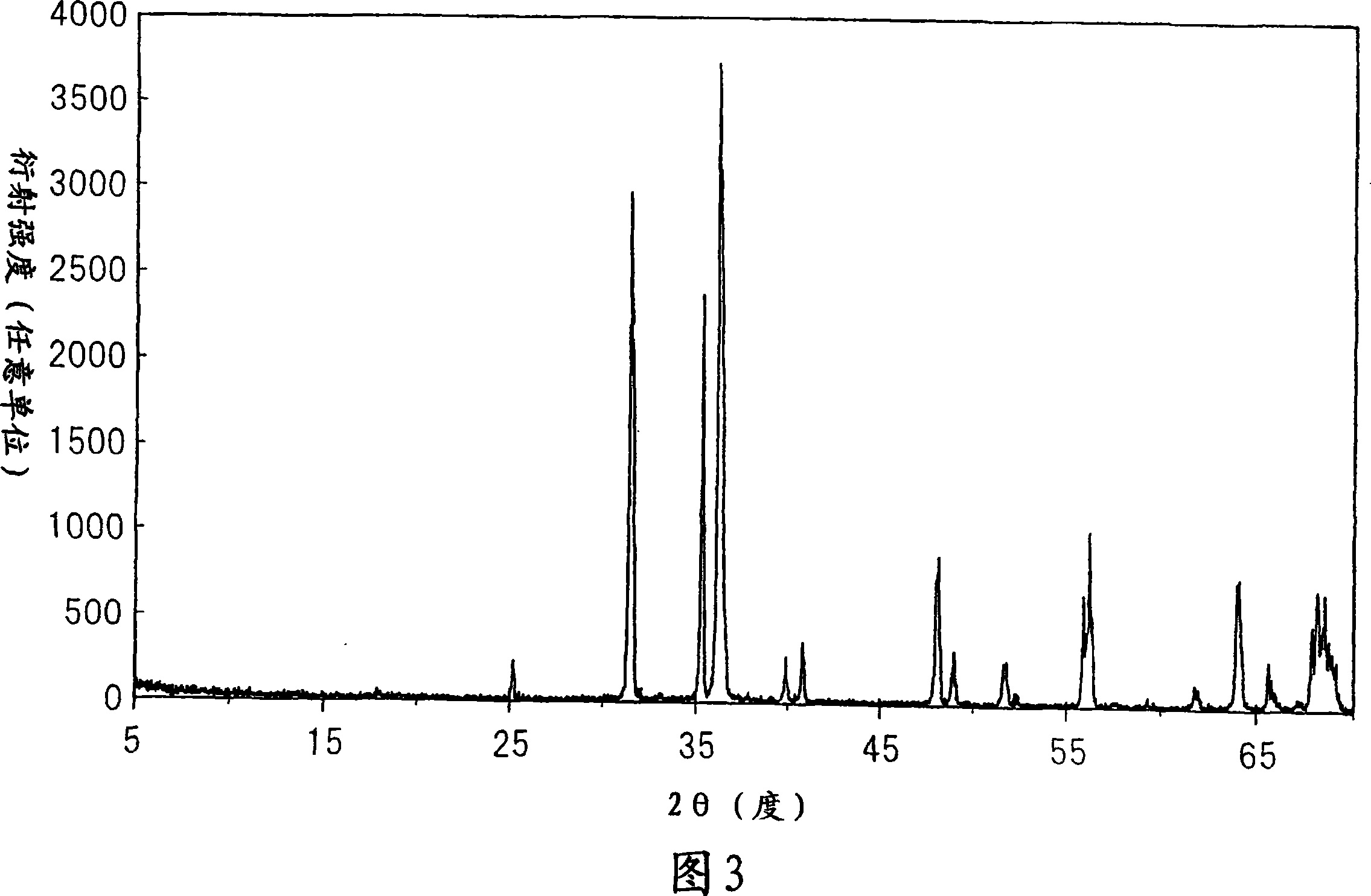
[0132]Prepare the same raw material as in Example 1, and in a nitrogen box with Ca 3 N 2 :AlN:Si 3 N 4 = Ratio of 20.0:60.0:20.0 (mol %) These raw materials were weighed, and EuN was further added to 100 parts by weight of the raw materials obtained in the above ratio so that Eu was 1.5 parts by weight. The resulting mixture was fired by the same method as in Example 1 to obtain a red fluorescent material. Figure 8 shows the X-ray diffraction pattern of this powder. Similar to Example 1, FIG. 9 shows an enlarged view of a portion where 2θ is 30°-42°. Here, for comparison, this red fluorescent material was compared with Comparative Example 9 (orthogonal CaAlSiN 3 ) and Example 1 (monoclinic CaAlSiN 3 )Compare. According to the comparison, it was found that the red fluorescent material of this example was a crystal similar to Example 1 and was monoclinic. Fig. 10 similarly shows a comparison of parts with 2Θ of 45°-70°. Also in this section, it was confirmed that the cr...
Embodiment 3
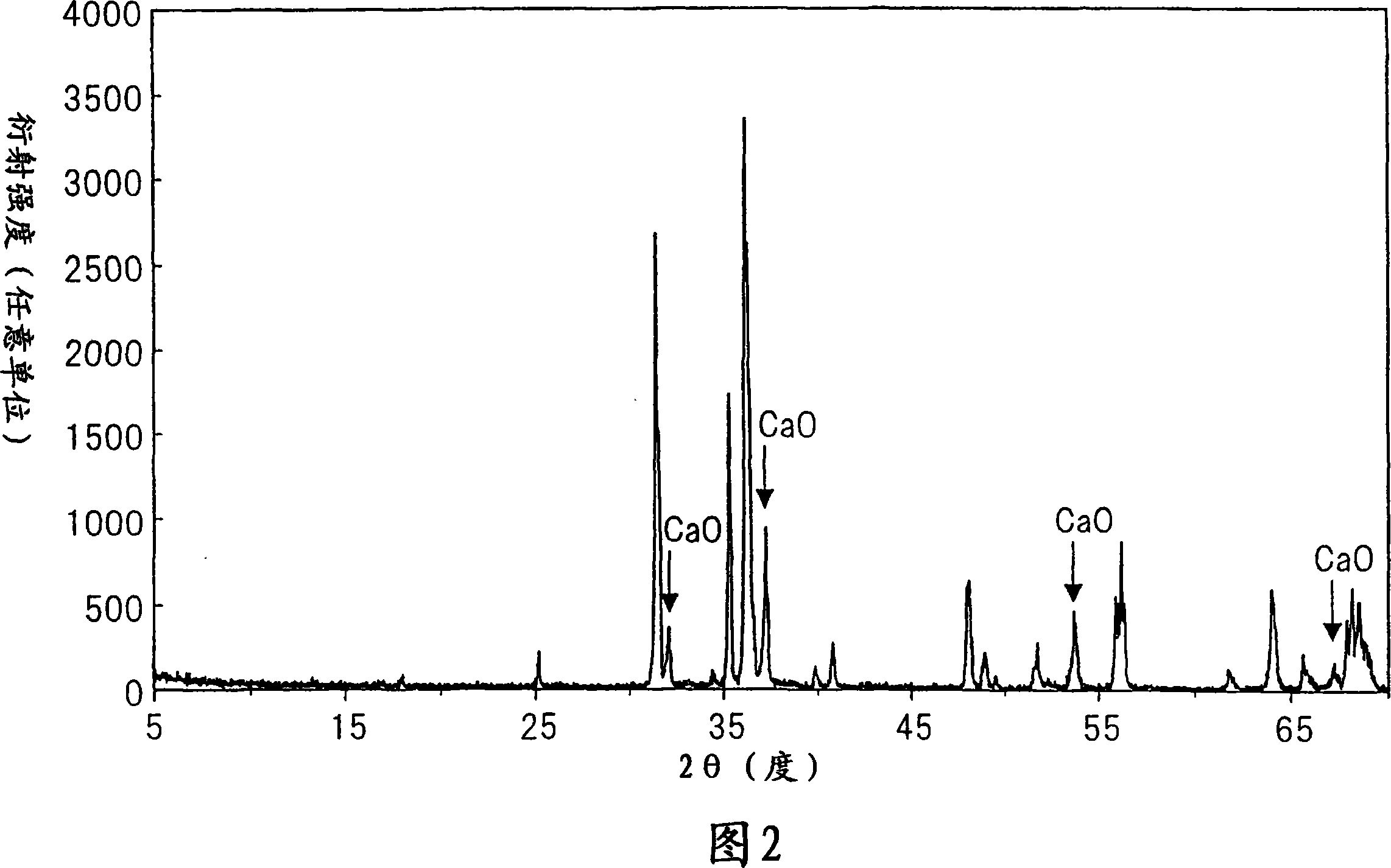
[0135] Prepare the same raw material as in Example 1, and in a nitrogen box with Ca 3 N 2 :AlN:Si 3 N 4 = Ratio of 60.0:30.0:10.0 (mol %) These raw materials were weighed, and EuN was further added to 100 parts by weight of the raw materials obtained in the above ratio so that Eu was 1.5 parts by weight. The resulting mixture was fired by the same method as in Example 1 to obtain a red fluorescent material. Figure 11 shows the X-ray diffraction pattern of this powder. The residue of CaO is remarkable, but the X-ray diffraction pattern except for CaO is the same as that of Example 1 and this red fluorescent material is found to be monoclinic CaAlSiN 3 . Since the red fluorescent material contained CaO, pickling was performed in the same manner as in Example 1 to remove CaO. The photoluminescence spectrum and the excitation spectrum were measured by the same method as in Example 1, and it was found that the photoluminescence spectrum and the excitation spectrum were almost...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| loose density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com