Anti-viral agent
An antiviral agent, hepatitis virus technology, applied in the direction of antiviral agent, antitumor drug, digestive system, etc., can solve problems such as no specific proof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
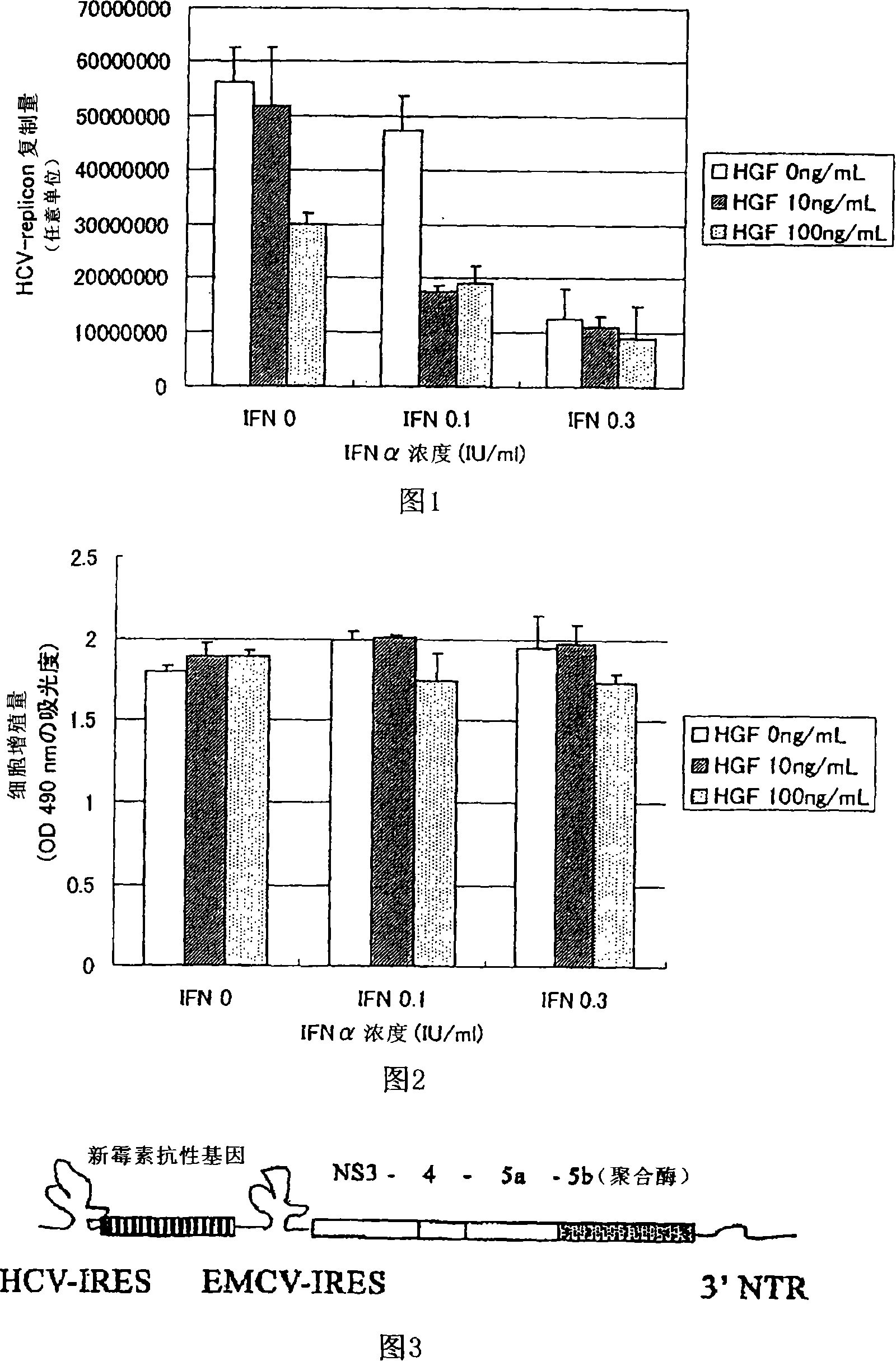
[0072] (Example 1) Effect of HGF Addition in HCV-Replicon Cells Relative to Inhibition of HCV-Replicon Replication of IFN
[0073] (Experimental materials and methods)
[0074] As a cell line for replicating HCV in vitro, a clone of HCV-replicon cells, #5-15 (purchased from ReBLikon GmbH), was used using the human hepatoma cell line Huh7 as a mother line. Suspend #5-15 in Dulbecco's Eagle's (Dalbetsukoi-イ-グル) MEM medium containing 2% bovine fetal serum, dilute at 1.5×10 4 / 100μl / well (well) was seeded on a 96-well plate. As a blank, a well (Day 0 ) in which cells were not seeded and only a medium was added was set. at 5% CO 2 After culturing in a humid incubator at 37°C for one day and night under gas, 50 μl of human recombinant IFNα (manufactured by BIOMEDICAL LABORATORIES, Cat. No. 11105-1, lot No. #2122) and / or human Recombinant HGF (manufactured by our company, Lot. 920629). The final concentration of IFNα is 0, 0.1, 0.3 international unit (IU) / ml, the final concentra...
Embodiment 2
[0081] (Example 2) Effects of IFN, HGF or combinations thereof on the proliferation of HCV-replicon cell lines
[0082] (Experimental materials and methods)
[0083] In order to investigate whether the decrease in the replication amount of HCV-replicon confirmed in Example 1 was due to the decrease in the number of cells itself, the following experiment was performed.
[0084] Suspend #5-15 in Dalubetsu Koi-Gru MEM medium containing 2% bovine fetal serum, and use 1.5×10 4 / 100μl / well seeded on a 96-well plate. As a blank, a well (Day 0 ) in which cells were not seeded and only a medium was added was set. at 5% CO 2 After culturing in a humid incubator at 37°C for one day and night under gas, 50 μl of human recombinant IFNα (manufactured by BIOMEDICAL LABORATORIES, Cat. No. 11105-1, lot No. #2122) and / or human Recombinant HGF (manufactured by our company, Lot. 920629). The final concentration of IFNα was 0, 0.1, 0.3 international unit (IU) / ml, the final concentration of HG...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com