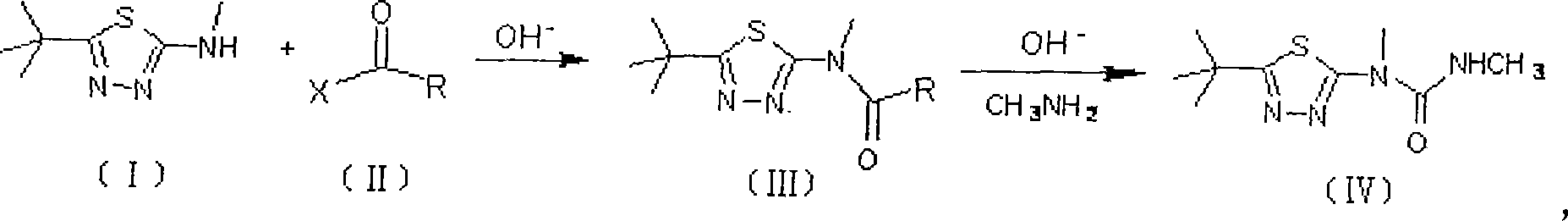
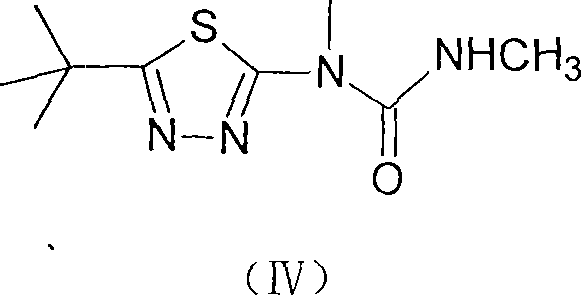
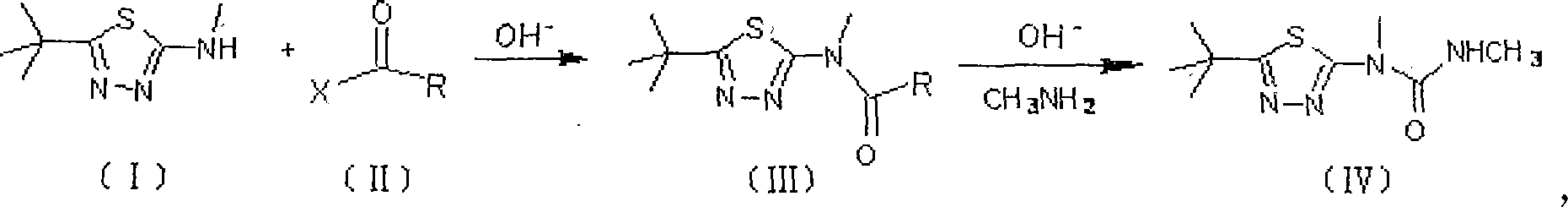
Method for preparing terbufos benzthiazuron
A technology of terbuthiuron and tert-butyl, which is applied in the field of preparation of the herbicide terbuthiuron, can solve problems such as unfavorable large-scale production and environmental pollution, achieve high yield and solve serious pollution effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Add 2-methylamino-5-tert-butyl-1,3,4-thiadiazole (I) 137g (0.8mol) and 800ml toluene in 2L reactor, slowly warm up to 50°C, turn off the heating system, and In the reaction system, 300 ml of toluene solution of 95 g of triphosgene (0.32 mol in terms of triphosgene) was added dropwise. After the dropwise addition, the temperature was raised to the reflux temperature, and the reaction was stopped after 3 hours of reflux reaction. After removing about 2 / 3 of the volume of toluene in the reaction system under reduced pressure, add 74 g of 40% methylamine aqueous solution (30 g in terms of methylamine, 0.96 mol) dropwise to the residue under ice-water cooling, and add dropwise within 0.5 hours. After completion, add 200 ml of 1 mol / L potassium carbonate (or NaOH) aqueous solution, remove the ice-water bath and wait for the reaction system to heat up naturally and slowly to 30-35° C. to continue the reaction for 4 hours. Filter and wash the filter cake twice with water. Sepa...
Embodiment 2
[0027]Add 17.1g (0.1mol) of 2-methylamino-5-tert-butyl-1,3,4-thiadiazole (I) and 100ml toluene into a 200ml reactor, slowly raise the temperature to 50°C, turn off the heating System, dropwise add 40ml of toluene solution of 12g of diphosgene (0.06mol in phosgene) to the reaction system, after the dropwise addition is completed, the temperature is raised to reflux temperature, and the reaction is continued for 3 hours under reflux to stop the reaction. After 2 / 3 of the volume of toluene in the reactant system was evaporated under reduced pressure, 9.5 g of 40% methylamine aqueous solution (0.12 mol in terms of methylamine) was added dropwise to the residue under ice-water cooling. After the addition was completed within 0.5 hours, , then add 25ml of 30ml of pyridine aqueous solution, remove the ice-water bath, wait for the reaction system to naturally and slowly heat up to 30°C-35°C, and continue the reaction for 4 hours to end. Filter and wash the filter cake twice with water...
Embodiment 3
[0029] Add 2-methylamino-5-tert-butyl-1,3,4-thiadiazole (I) 35g (0.2mol) and 300ml toluene in a 500ml reactor, and feed into the reaction system within 2 hours at room temperature 25g (0.12mol) of phosgene will be slowly raised to the reflux temperature after passing through, and the reaction will be stopped after continuing the reaction under reflux for 3 hours. After recovering 2 / 3 of the volume of toluene in the reactant system under reduced pressure, add 20 g of 40% methylamine aqueous solution (0.24 mol in terms of methylamine) dropwise to the residue under ice-cooling, the dropwise addition is completed within 0.5 hours, and then add 15ml of triethylamine, remove the ice-water bath, and after the reaction system naturally warms up to room temperature, slowly raise the temperature of the system to 30°C-35°C, and continue the reaction for 4 hours to end. Filter and wash the filter cake twice with water. Separate the toluene in the filtrate and recover the toluene to conce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com