Process of manufacturing viral vaccines in suspension avian embryonic derived stem cell lines
A technology of stem cells and embryos, applied in the field of virus vaccine development and preparation, can solve the problems of heavy workload, long cycle, and large resource consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0159] Example 1: Method for Constructing EBx(R) Cell Line Derivatives
[0160] Methods for the construction of avian embryo-derived stem cells EBx(R) were previously described in WO03 / 076601 and WO05 / 007840. Briefly, the method for EBx(R) cell line construction comprises the following steps:
[0161] a) Isolation, cultivation and expansion of avian cells in complete medium containing all growth factors necessary for growth in the presence of a mouse fibroblast feeder layer (preferably inactivated) supplemented with animal serum, preferably Avian embryonic stem cells;
[0162] b) passaging by improving the medium or completely removing the factors, the serum and the feeder layer to accumulate cells;
[0163] c) establishment of adherent and non-adherent avian cell lines capable of proliferating in basal medium without exogenous growth factors, inactivated feeder layers, and low or no serum;
[0164] If the minimal medium of step c) still contains low levels of serum (e.g. a...
Embodiment 2
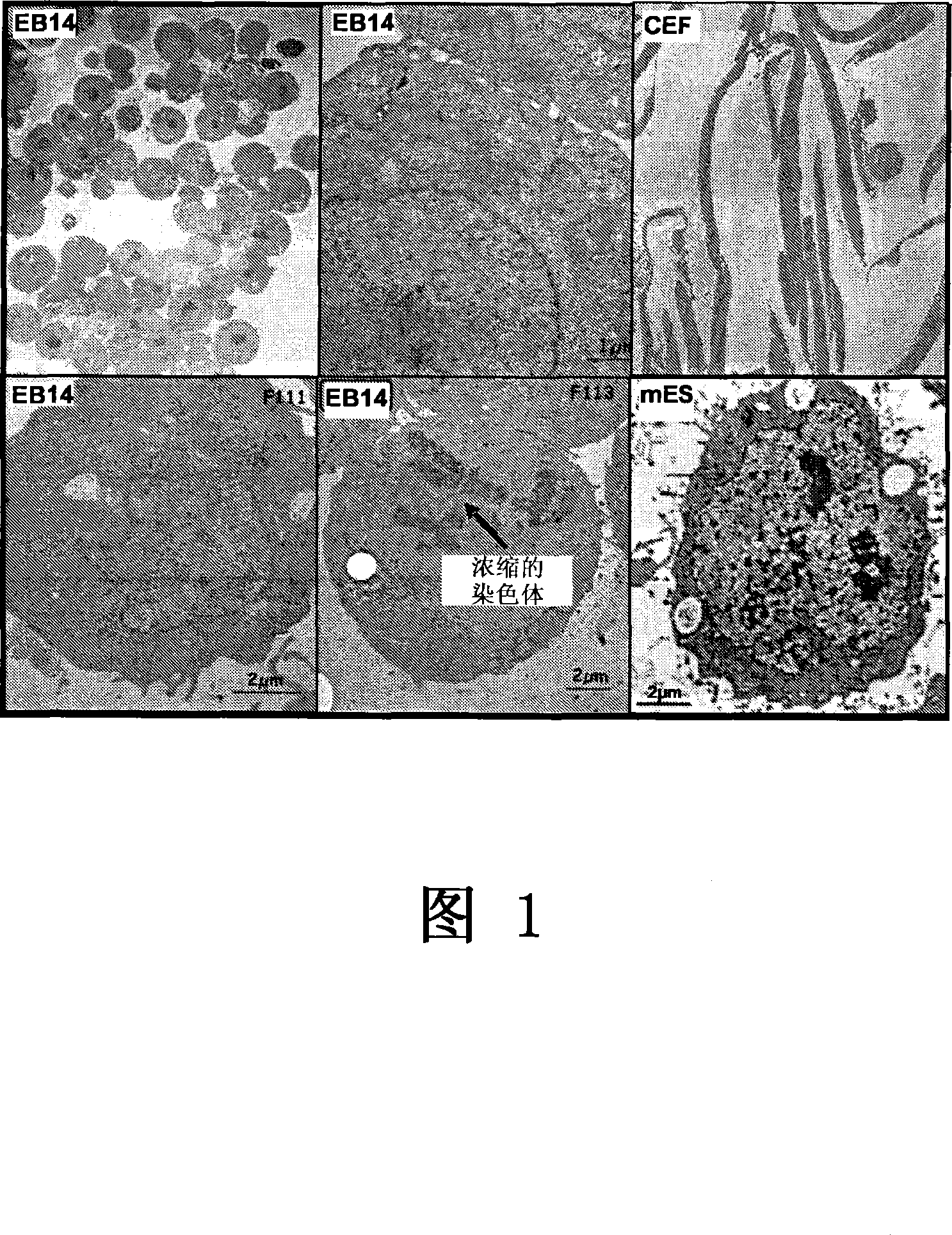
[0199] Example 2: Characteristics of EB14 cells
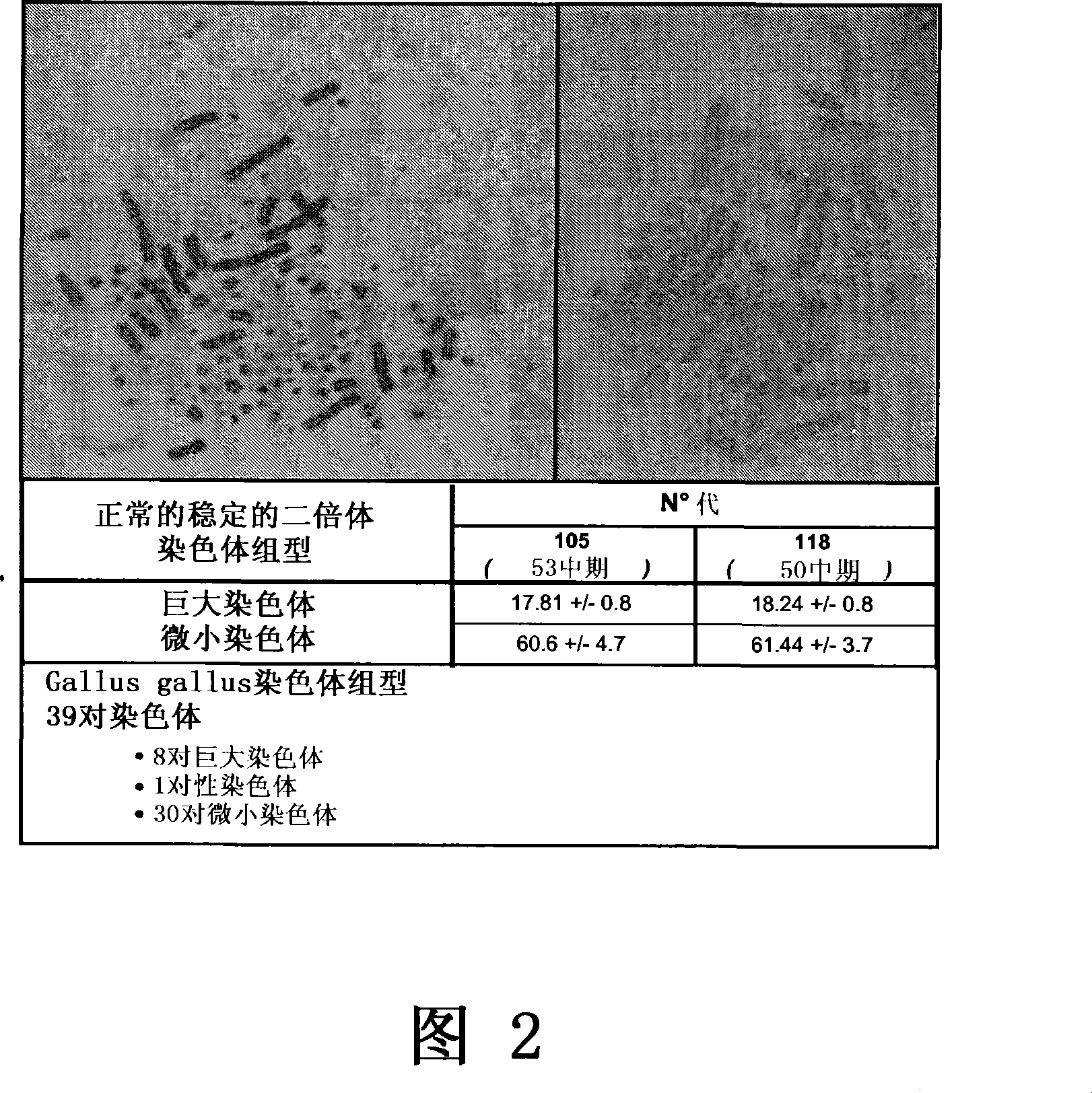
[0200] 2.1 Karyotype of EB14 cells
[0201] The laboratory of Pr. Michel Franck has analyzed the karyotype of EB14 cells (Unite de zootechnie, ethnologie et economie rurale, Ecole Nationale Veterinaire, 1 avenue Bourgelat, 69280 Marcy I'Etoile, France).
[0202] Karyotyping of EB14 cells at two different passages (105 and 118) was performed using standard techniques well known to those skilled in the art. As expected, EB14 cells at passages 105 and 118 were diploid karyotypes (Fig. 2):
[0203] Generation 105: number of chromosomes = 78 (mean: 78.41 - standard deviation: 4.951 for the 53 studied metaphase chromosomes).
[0204] Generation 118: number of chromosomes = 79 (mean: 79.68 - standard deviation: 3.733 for the 50 studied metaphase chromosomes).
[0205] The chicken genome contains two types of chromosome pairs: giant and tiny. The analysis of the 115th generation shows that the number of giant chromosomes is 18, wit...
Embodiment 3
[0215] Example 3: MVA production in EB14 cells
[0216] 3.1 Materials and methods
[0217] A recombinant MVA virus encoding green fluorescent protein was used in the experiment. The titer of infectious MVA-GFP virus was determined using DF-1 cells. Simply put, with 15×10 3 The density of cells / well Seed cells in 96-well flat-bottom culture plate, the medium used is the DMEM medium (Biowhittaker) that has added 5% fetal calf serum (FCS) (SAFC) and 2mM L-glutamine (Biowhittaker) . After 24 hours, infect the cells with 10-fold serially diluted samples of DMEM medium at 37 °C, 5% CO 2 Incubate for 1 week under humidified conditions. Viral infection was determined by microscopic observation of spherocytopathy (CPE) and exposure of infected cells to UV light. Then, the TCID50 titer was calculated using the method of Reed and Muench (1938, Asimple method of estimating fifty percent endpoints. Am. J. Hyg. 27, 493-97).
[0218] 3.2 - Infection in tissue culture flasks
[0219] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com