Low birefringent copolymer
A technology of copolymer and acrylic acid, applied in instruments, optics, nonlinear optics, etc., can solve the problems of easy thermal yellowing and coloring of resins, and achieve the effects of excellent transparency, excellent heat resistance and low coloring.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0094]
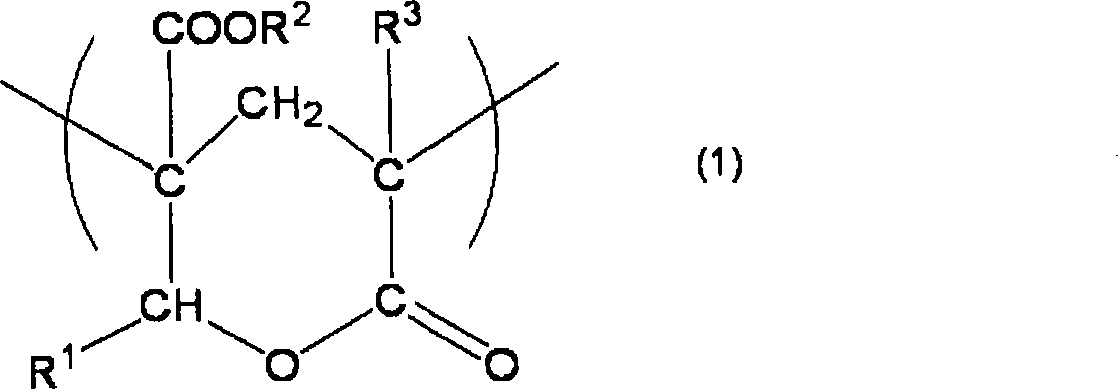
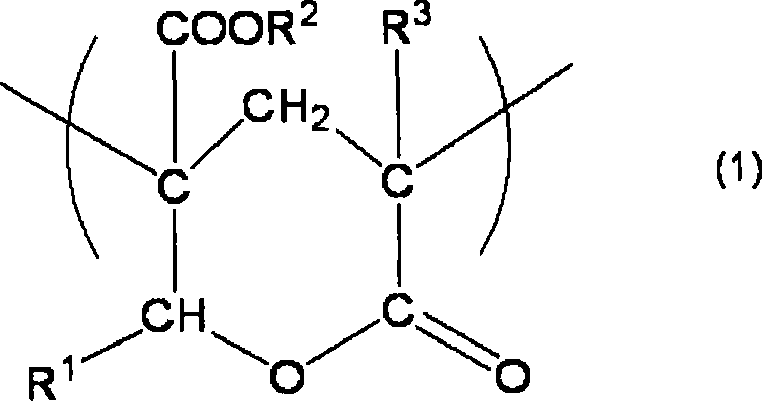
[0095] The preparation of the acrylic copolymer is not particularly limited. First, in the case of an acrylic copolymer having a structural unit providing a negative phase difference and a lactone ring structure as a structural unit providing a positive phase difference in the molecular chain, it can be obtained by The acrylic copolymer (a) having a structural unit providing a negative phase difference in the molecular chain and having a hydroxyl group and an ester group in the molecular chain is obtained by the polymerization process, and then the obtained acrylic copolymer (a) is heat-treated, It is obtained by performing a cyclocondensation process of introducing a lactone ring structure providing a positive retardation into an acrylic copolymer.
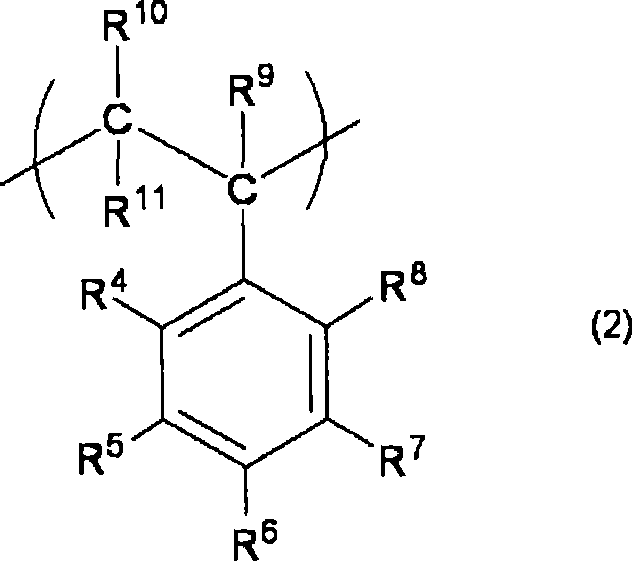
[0096] At this time, in the polymerization step, for example, the monomer component represented by the following formula (6) and the monomer represented by the following formula (7) can be obtained through the polymeriz...
Embodiment 1
[0228] First, add 7 kg of methyl methacrylate, 2 kg of 2-(hydroxymethyl) methyl acrylate, and 1 kg of styrene , 10kg of methyl isobutyl ketone, 5g of n-dodecyl mercaptan.
[0229] Nitrogen gas was introduced into the reaction vessel, and the temperature was raised to 105° C., 5 g of tert-amyl 3,5,5-trimethylhexanoate was added as a polymerization initiator under reflux, and at the same time, 230 g of formic acid was added dropwise over 2 hours. 10 g of tert-amyl 3,5,5-trimethylhexanoate was dissolved in methyl isobutyl ketone, solution polymerization was carried out at about 105-120° C. under reflux, and aging was carried out for 4 hours.
[0230] In the obtained acrylic copolymer solution, add 30 g of stearyl phosphate / dioctadecyl phosphate mixture (PhoslexA-18, manufactured by Sakai Chemical Industry Co., Ltd.), under reflux at about 90- The cyclocondensation reaction was carried out at 120° C. for 5 hours. Then, the obtained acrylic copolymer solution is introduced into t...
Embodiment 2
[0261] First, add 7950 g of methyl methacrylate, 1500 g of 2-(hydroxymethyl) methyl acrylate, and 550 g of styrene in a reaction vessel with a stirring device, a temperature sensor, a cooling pipe, and a nitrogen inlet pipe with a capacity of 30 L. , 10,000 g of toluene.
[0262] Nitrogen gas was introduced into the reaction vessel, and the temperature was raised to 105° C., 12 g of tert-amyl peroxyisononanoate as a polymerization initiator was added under reflux, and 24 g of peroxide dissolved in 136 g of toluene was added dropwise over 2 hours. A solution of tert-amyl isononanoate was oxidized and solution polymerized at about 105-110°C under reflux, followed by a further 4 hours of aging.
[0263] 10 g of octyl phosphate (Phoslex A-8, manufactured by Sakai Chemical Industry Co., Ltd.) was added to the obtained acrylic copolymer solution, and cyclocondensation reaction was carried out at about 120° C. under pressure for 5 hours. Then, the obtained acrylic copolymer solution...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com