New pyridine analogues
A compound, C1-C12 technology, applied in the field of pyridine compounds, can solve the problems of acute thrombosis and high morbidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
no. 2 approach
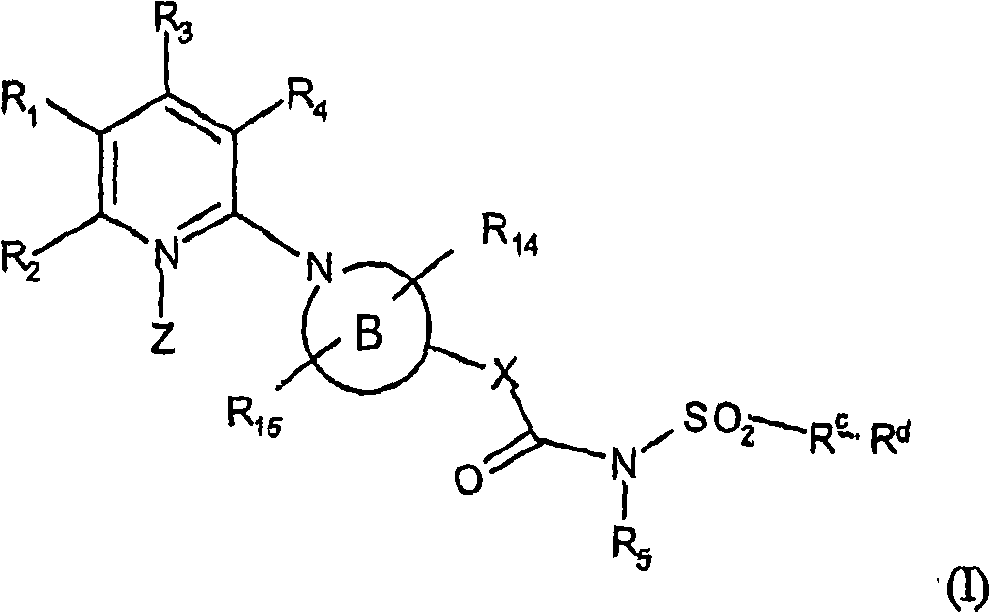
[0092] A second embodiment of formula I is defined as follows:
[0093] R 1 means R 6 OC(O), R 7 C(O), R 16 SC(O), R 17 S, R 18 C(S) or group gII:
[0094]
[0095] R 2 Indicates H, CN, NO 2 , (C optionally interrupted by oxygen and / or optionally substituted by OH, aryl, cycloalkyl, heterocyclyl or one or more halogen (F, Cl, Br, I) atoms 1 -C 6 ) alkyl; In addition, R 2 represents (C optionally substituted by one or more halogen (F, Cl, Br, I) atoms 1 -C 6 ) alkoxy; In addition, R 2 Indicates (C 3 -C 6 ) cycloalkyl, hydroxyl (C 1 -C 6 ) Alkyl, (C 1 -C 6 ) Alkyl C (O), (C 1 -C 6 ) Alkylthio C (O), (C 1 -C 6 ) Alkyl C(S), (C 1 -C 6 ) alkoxy C (O), (C 3 -C 6 ) cycloalkoxy, aryl, aryl C (O), aryl (C 1 -C 6 ) Alkyl C (O), heterocyclyl, heterocyclyl C (O), heterocyclyl (C 1 -C 6 ) Alkyl C (O), (C 1 -C 6 ) Alkylsulfinyl, (C 1 -C 6 ) Alkylsulfonyl, (C 1 -C 6 ) Alkylthio, (C 3 -C 6 ) cycloalkylthio, arylsulfinyl, arylsulfonyl, arylthio, aryl (C...
no. 3 approach
[0114] A third embodiment of formula I is defined as follows:
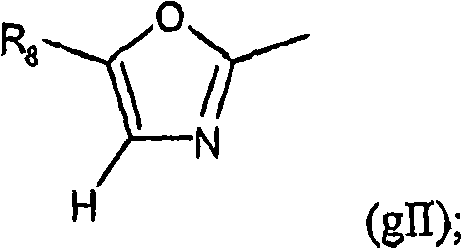
[0115] R 1 means R 6 OC(O), R 16 SC(O) or group gII:
[0116]
[0117] R 2 Indicates H, CN, NO 2 , (C optionally interrupted by oxygen and / or optionally substituted by OH, aryl, cycloalkyl, heterocyclyl or one or more halogen (F, Cl, Br, I) atoms 1 -C 6 ) alkyl; In addition, R 2 represents (C optionally substituted by one or more halogen (F, Cl, Br, I) atoms 1 -C 6 ) alkoxy; In addition, R 2 Indicates (C 3 -C 6 ) cycloalkyl, hydroxyl (C 1 -C 6 ) Alkyl, (C 1 -C 6 ) Alkyl C (O), (C 1 -C 6 ) Alkylthio C (O), (C 1 -C 6 ) Alkyl C(S), (C 1 -C 6 ) alkoxy C (O), (C 3 -C 6 ) cycloalkoxy, aryl, aryl C (O), aryl (C 1 -C 6 ) Alkyl C (O), heterocyclyl, heterocyclyl C (O), heterocyclyl (C 1 -C 6 ) Alkyl C (O) or formula NR a(2) R b(2) group, where R a(2) and R b(2) Independently represent H, (C 1 -C 6 ) Alkyl, (C 1 -C 6 ) alkyl C (O), or R a(2) and R b(2) together with a nitrogen atom rep...
no. 4 approach
[0132] A fourth embodiment of formula I is defined as follows:
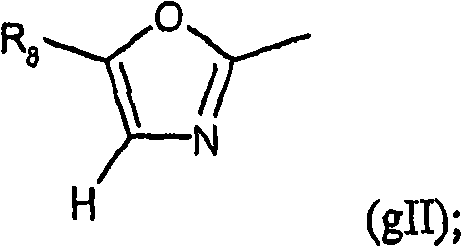
[0133] R 1 means R 6 OC(O), R 16 SC(O) or group gII:
[0134]
[0135] R 2 represents H or (C optionally interrupted by oxygen and / or optionally substituted by OH, aryl, cycloalkyl, heterocyclyl or one or more halogen (F, Cl, Br, I) atoms 1 -C 6 ) alkyl; In addition, R 2 Expression NR a(2) R b(2) group, where R a(2) and R b(2) Independently represent H, (C 1 -C 6 ) Alkyl, (C 1 -C 6 ) alkyl C (O), or R a(2) and R b(2) together with a nitrogen atom represents piperidine, pyrrolidine, azetidine or aziridine;
[0136] R 3 Represents H or formula NR a(3) R b(3) group, where R a(3) and R b(3) Independently represent H, (C 1 -C 6 ) Alkyl, (C 1 -C 6 ) alkyl C (O), or R a(3) and R b(3) together with a nitrogen atom represents piperidine, pyrrolidine, azetidine or aziridine;
[0137] R 4 Indicates CN, halogen (F, Cl, Br, I), in addition, R 4 Indicates (C 1 -C 6 ) Alkyl C (O), (C 1 -C 6 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Yield | aaaaa | aaaaa |
| Yield | aaaaa | aaaaa |
| Yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com