Novel asymmetric synthesis of (S)-(+)-3-(aminomethyl)-5-methylhexanoic acid
A carbamoyl and methyl technology, applied in the field of -pregabalin and -pregabalin intermediates, synthesis of -3--5-methylhexanoic acid, can solve the problem of product loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
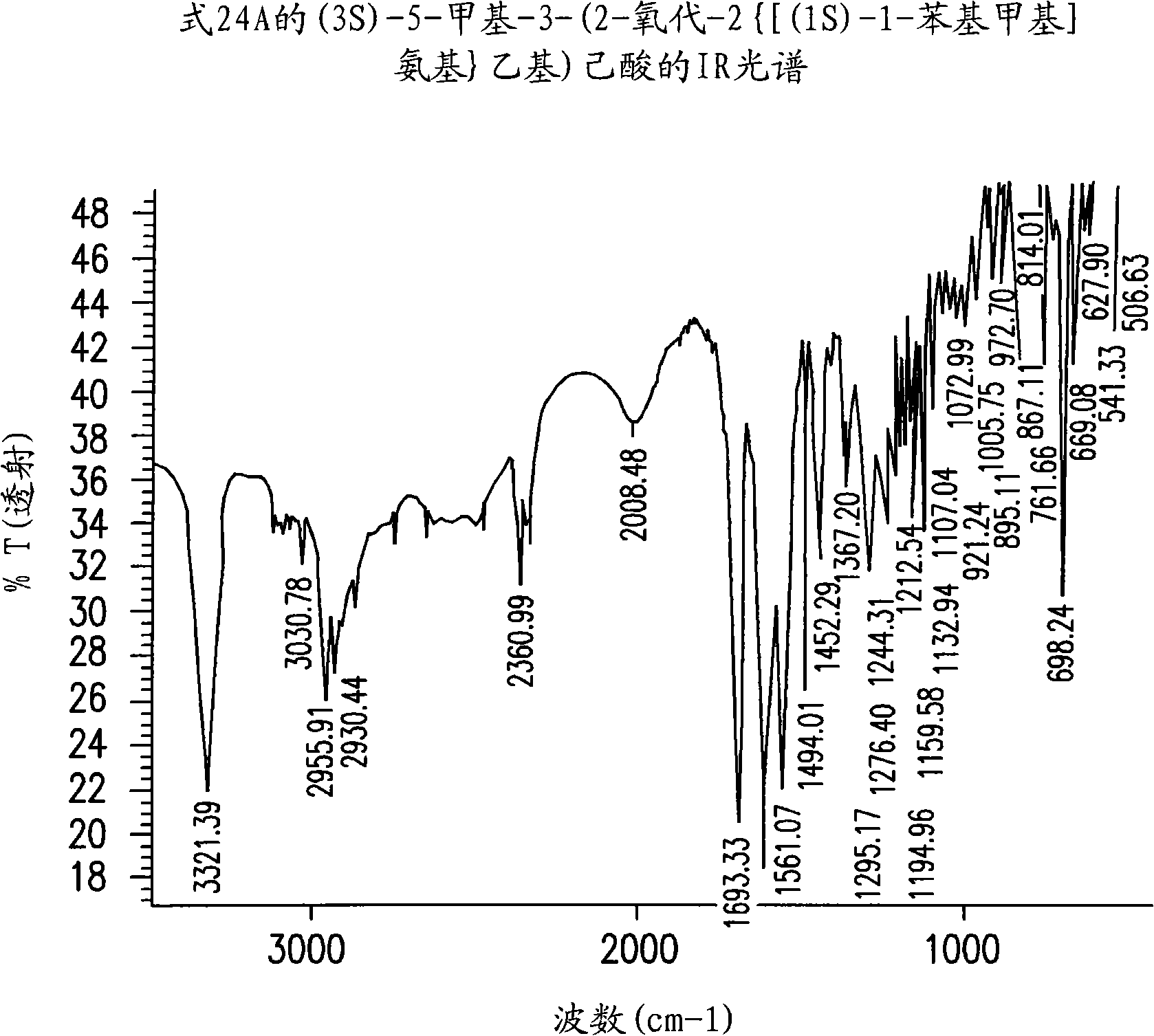
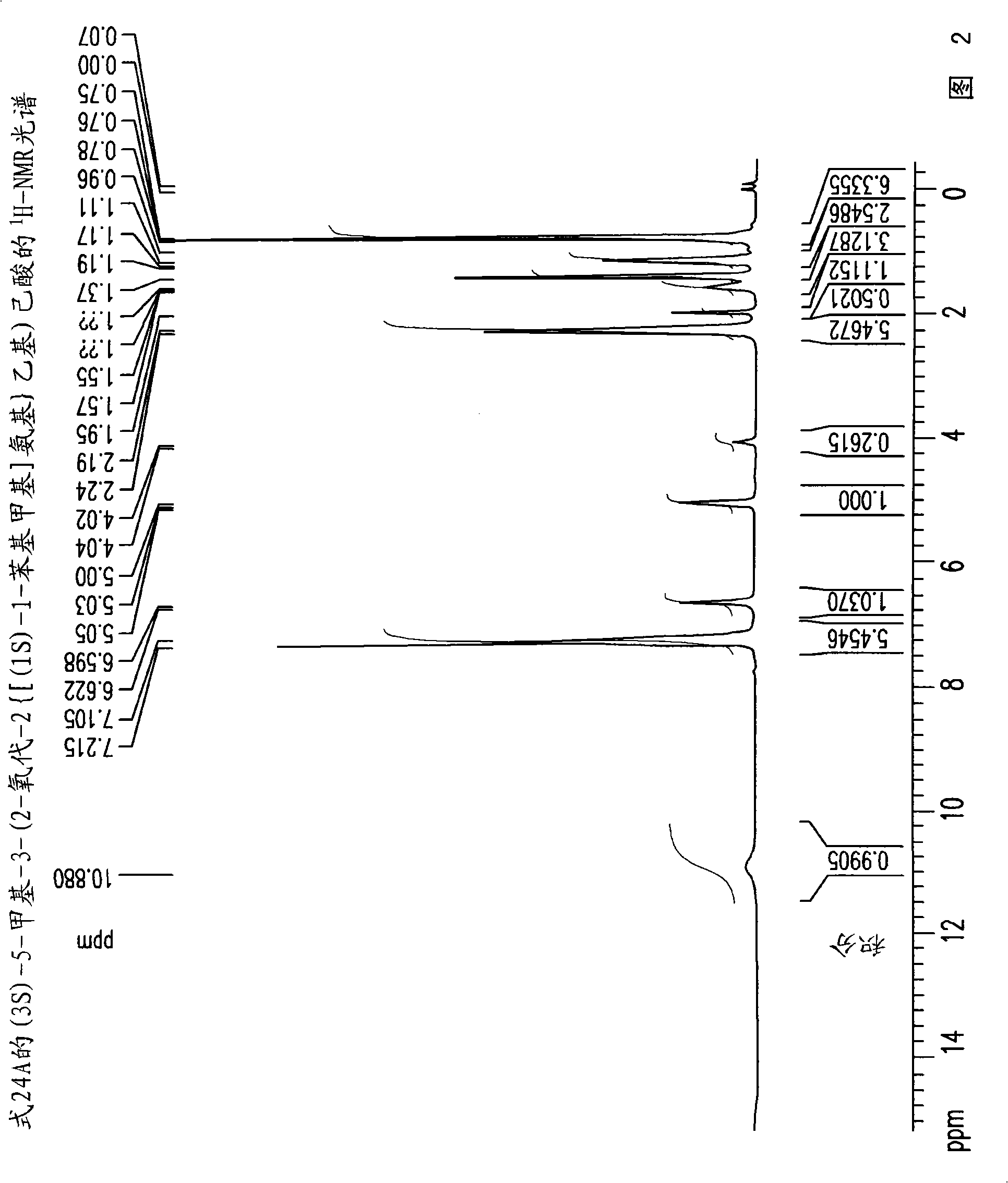
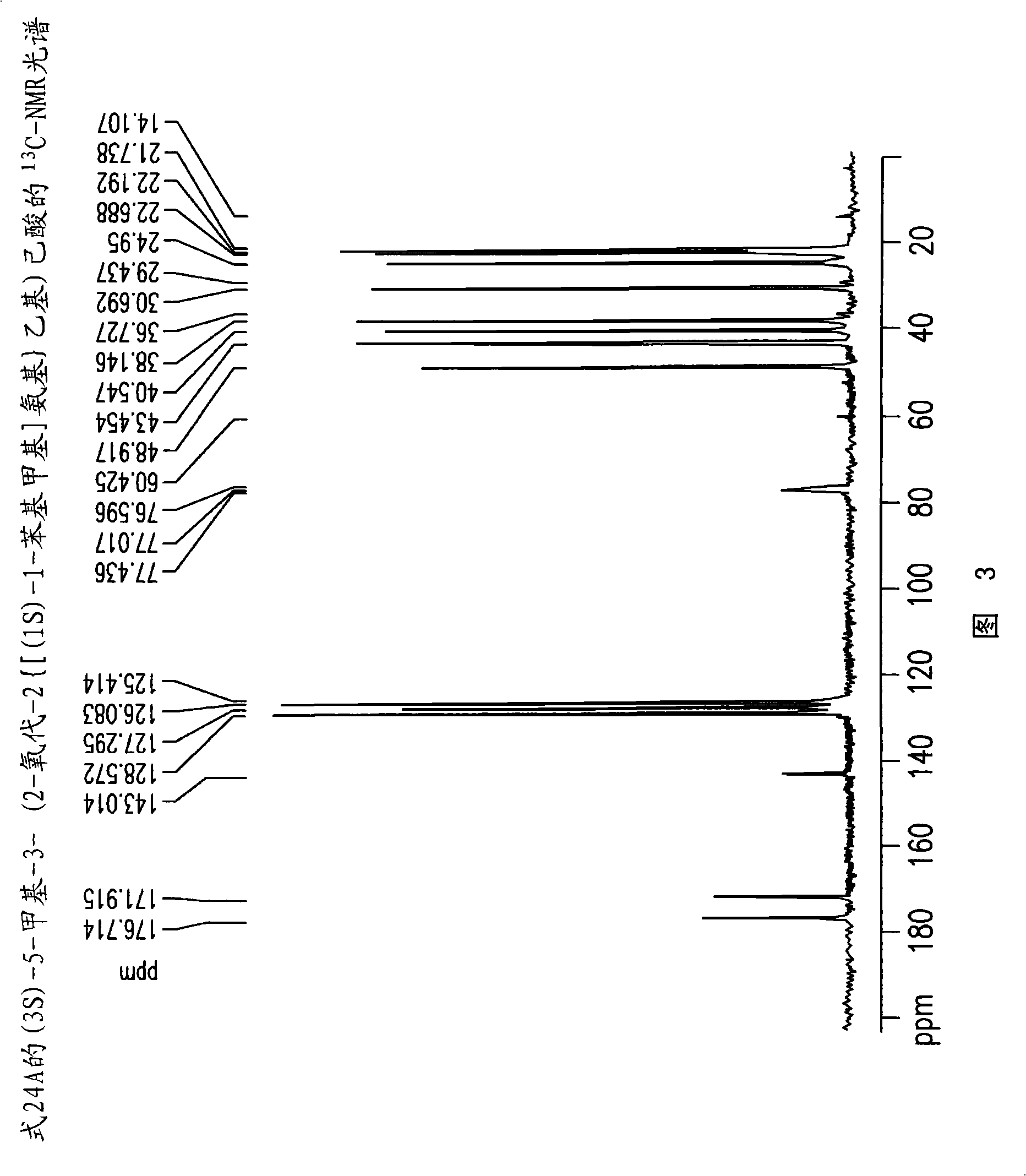
[0267] Example 1: (3S)-5-methyl-3-(2-oxo-2{[(1S)-1-phenylethyl]amino}ethyl)hexanoylation Preparation of compound (24)
[0268] Toluene (400ml), (S)-(-)-phenethylamine (142.35g, 1.1764mol) and 4-dimethyl Aminopyridine (0.7176 g, 0.0059 mol). The mixture was cooled to a temperature of -10°C to -15°C, followed by the addition of a solution of 3-isobutylglutaric anhydride (100 g, 0.59 mol) in toluene (100 ml) over a period of 45-60 minutes, and at -10°C Stir for an additional 1.5-2 hours to a temperature of -15°C. The mixture was then extracted with 10% aqueous NaOH (500ml) and the aqueous phase was washed with toluene (1 x 250ml). The pH of the aqueous phase was adjusted to 2-2.5 by adding aqueous hydrochloric acid (1-12N). The aqueous phase was further extracted with toluene (1 x 800ml) at a temperature of 70-80°C. The toluene layer was washed with 10% sodium chloride solution (700ml) at 70-80°C, followed by crystallization to give 125g (73.0% yield) of (3S)-5-methyl-3-(...
Embodiment 2
[0269] Example 2: (3S)-5-methyl-3-(2-oxo-2{[(1S)-1-phenylethyl]amino}ethyl)hexanoylation Preparation of compound (24)
[0270] Toluene (400ml), (S)-(-)-phenylethylamine (38.59g, 0.0.319 moles) and 4-bis Methylaminopyridine (0.358 g, 0.0029 moles). The mixture was cooled to a temperature of -40°C to -50°C, then a solution of 3-isobutylglutaric anhydride (50 g, 0.294 mol) in toluene (100 ml) was added over a period of 45-60 minutes and heated at -40°C Stir for an additional 1.5-2 hours to a temperature of -50°C. The mixture was then extracted with 3.5-4.0% aqueous NaOH (1000ml), and the aqueous phase was washed with toluene (1 x 250ml). The pH of the aqueous phase was adjusted to 2-2.5 by adding aqueous hydrochloric acid (1-12N). The aqueous phase was further extracted with ethyl acetate (1 x 300ml and 1 x 100ml), then the combined ethyl acetate extracts were dried over anhydrous sodium sulfate, and the solvent was removed to obtain a residue. The residue was crystallize...
Embodiment 3
[0271] Example 3: (3S)-5-methyl-3-(2-oxo-2{[(1S)-1-phenylethyl]amino}ethyl)hexanoylation Preparation of compound (24)
[0272] Toluene (1000ml), (S)-(-)-phenylethylamine (266.9g, 2.206mol) and 4-dimethyl Aminopyridine (1.79 g, 0.0147 mol). The mixture was cooled to a temperature of -40°C to -50°C, followed by the addition of a solution of 3-isobutylglutaric anhydride (250 g, 1.471 mol) in toluene (250 ml) over a period of 45-60 minutes, and at -40°C Stir for an additional 1.5-2 hours to a temperature of -50°C. The mixture was then extracted with 3.5-4.0% aqueous NaOH (2350ml), and the aqueous phase was washed with toluene (1 x 250ml). The pH of the aqueous phase was adjusted to 2-2.5 by adding aqueous hydrochloric acid (1-12N). The aqueous phase was further extracted with ethyl acetate (1 x 1250ml and 1 x 500ml), then the combined ethyl acetate extracts were dried over anhydrous sodium sulfate, and the solvent was removed to obtain a residue. The residue was crystalliz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com