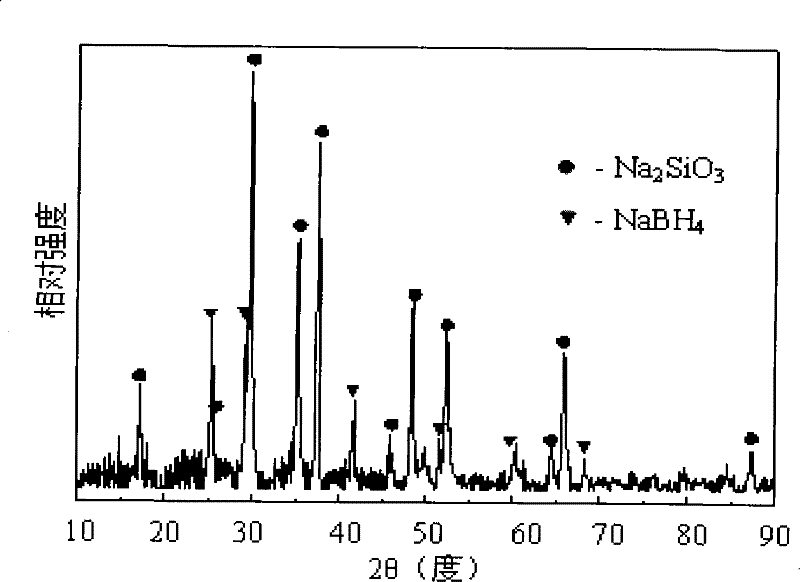
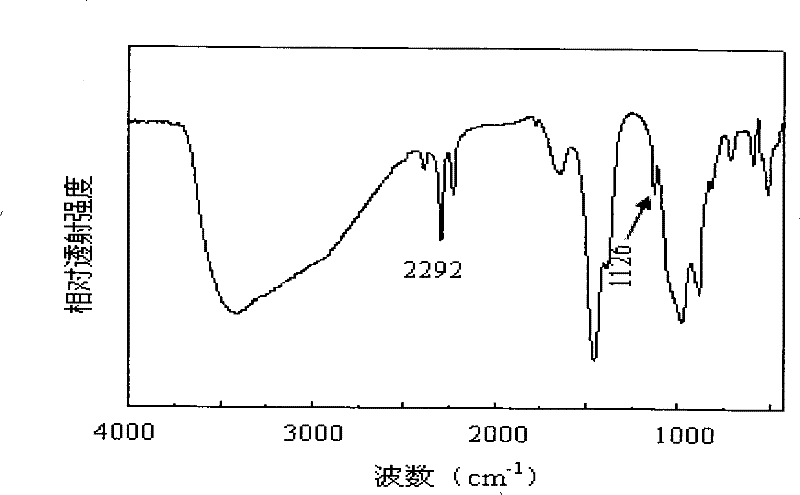
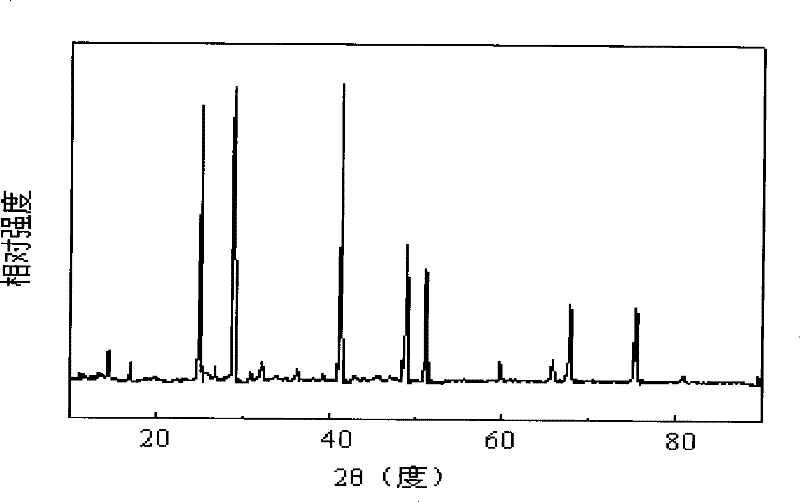
Method for preparing sodium borohydride
A technology for sodium borohydride and hydride, applied in the field of compound preparation, can solve problems such as high equipment requirements, increase production costs, etc., and achieve the effects of simple process, avoiding strict requirements, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Remove mineral oil from industrial-grade sodium hydride (Shanghai Senhao Fine Chemical Co., Ltd.), weigh 9.6 g under nitrogen protection for use; remove water from sodium metaborate (Sinopharm Group), crush it to 100-200 mesh, and weigh 6.58 g g for use; crush silicon dioxide (Sinopharm Group) to 100-200 mesh, and weigh 12g. The reactants weighed above were then mechanically mixed for 2 hours under nitrogen protection. Under the protection of nitrogen, the above mixture was rolled into a block with a pressure of 20 MPa. Then, under the protection of 0.1atm hydrogen, the temperature was raised to 510°C at a rate of 10°C / min, calcined for 8 hours, and cooled to room temperature with the furnace. The reaction product was pulverized, and 150 ml of isopropylamine was added as an extractant, extracted with a Soxhlet extractor for 2 h, and then the isopropylamine was recovered by distillation to obtain 2.97 g of sodium borohydride, and the yield was 78.6% of the theoretical v...
Embodiment 2
[0026] Remove mineral oil from industrial-grade sodium hydride (Shanghai Senhao Fine Chemical Co., Ltd.), weigh 12g under nitrogen protection for use; remove water from sodium metaborate (Sinopharm Group), crush it to 100-200 mesh, and weigh 6.58g Stand-by; crush silicon dioxide (Sinopharm Group) to 100-200 mesh, and weigh 12g. The reactants weighed above were then mechanically mixed for 2 hours under nitrogen protection. Under the protection of nitrogen, the above mixture was rolled into a block with a pressure of 20 MPa. Then, under the protection of high-purity nitrogen at normal pressure, the temperature was raised to 500°C at a rate of 5°C / min, calcined for 8 hours, and cooled to room temperature with the furnace. The reaction product was pulverized, and 150 ml of isopropylamine was added as an extractant, extracted with a Soxhlet extractor for 2 h, and then the isopropylamine was recovered by distillation to obtain 2.876 g of sodium borohydride, and the yield was 76.1% ...
Embodiment 3
[0028] Remove mineral oil from industrial-grade sodium hydride (Shanghai Senhao Fine Chemical Co., Ltd.), and weigh 9.6g under nitrogen protection for use; remove water from sodium metaborate (Sinopharm Group), crush it to 100-200 mesh, and weigh 6.58g g for use; crush silicon dioxide (Sinopharm Group) to 100-200 mesh, and weigh 12g. The reactants weighed above were then mechanically mixed for 2 hours under nitrogen protection. Under the protection of nitrogen, the above mixture was rolled into a block with a pressure of 20 MPa, and the block was crushed to 50-100 mesh again. Then, under the protection of 2atm hydrogen, the temperature was raised to 480°C at a rate of 10°C / min, calcined for 6 hours, and cooled to room temperature with the furnace. The reaction product was pulverized, and 150 ml of isopropylamine was added as an extractant, extracted with a Soxhlet extractor for 2 h, and then the isopropylamine was recovered by distillation to obtain 3.13 g of sodium borohydri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com