2-pyrimindinyloxy (pyrimindinylthio) benzoxy enoates compound and application thereof
A technology of pyrimidinylthiobenzoyl enoate and pyrimidinyloxybenzoyl enoate is applied in the field of 2-pyrimidinyloxybenzoyl enoate compounds, and can solve problems that do not involve 2-pyrimidinyloxy( Thio) base benzoic acid base enoate compounds and other issues
Active Publication Date: 2010-09-01
SHENYANG SINOCHEM AGROCHEMICALS R&D CO LTD
View PDF4 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
The prior art does not relate to the preparation and application of 2-pyrimidinyloxy (thio) base benzoic acid base enoate compounds as shown in the present invention
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Synthetic example
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
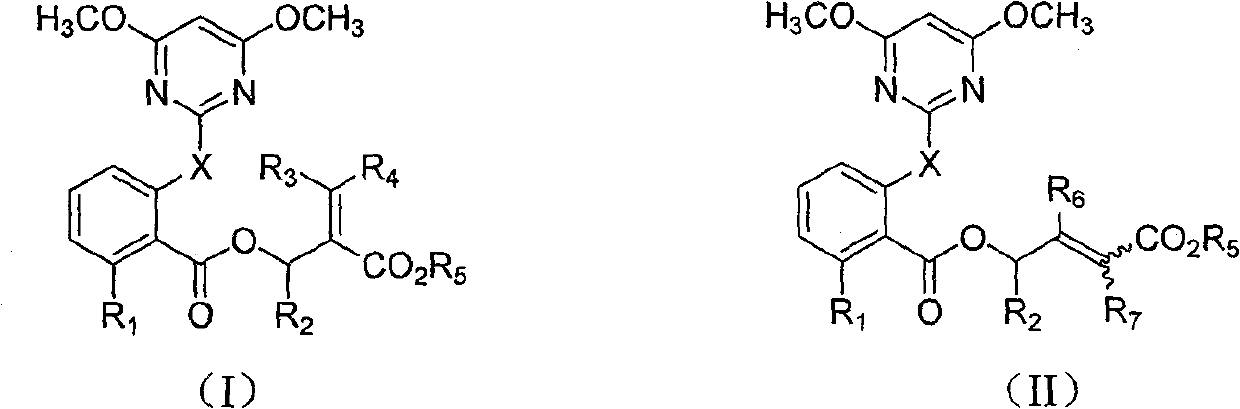
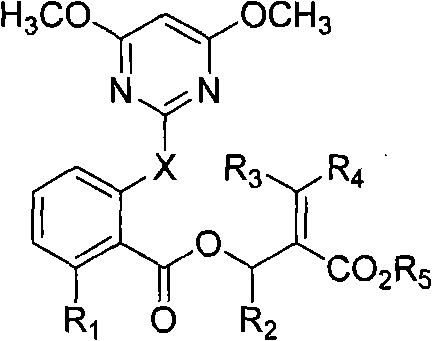
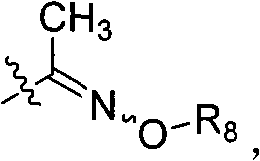
The invention discloses 2-pyridine-oxyl(sulfide) benzoyloxy enoates compound with general formula(I, II); wherein X is H or S; R1 is H, halogen, or 4,6- dimethoxy pyrimidine-2- radicel or one of the following groups; methyls on N-O bonds and double bonds are in maleinoid form or anti form; R2,R3,R4,R6,R7 are selected from H or alkyl from C1-C6; R5 is alkyl from C1-C6, alcoxyl-alkyl from C2-C6, alkenyl from C3-C6 or alkynyl from C3-C6; radicals of R6 and R7 are in maleinoid form or anti form; R8 is alkyl from C1-C6, alkenyl from C3-C6 or alkynyl from C3-C6. The compound (I, II)is of activity in weeding and is safe to crops like soybean, cotton, earthnut, cole, corn, etc.
Description
technical field The invention belongs to the field of herbicides, and in particular relates to a 2-pyrimidinyloxy (thio) benzoyl enoate compound and an application thereof. Background technique Due to the succession and change of weed populations and the emergence and rapid development of resistance to chemical pesticides, people's awareness of ecological environment protection has been continuously strengthened. The emphasis on the issue of whereabouts continues to increase. With the gradual reduction of the world's arable land, the continuous growth of the population and the increase in the demand for food, people are forced to rapidly develop agricultural production technology, improve and improve the farming system, and need to continuously invent new and improved herbicidal compounds and compositions. EP223406 reported that certain 2-pyrimidinyloxy (thio) benzoate compounds have herbicidal activity and are safe to peanuts and sunflowers. CN1749236A and CN1927811A dis...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): C07D239/60A01N43/54A01P13/00
Inventor 李斌冀海英崔东亮白丽萍张弘
Owner SHENYANG SINOCHEM AGROCHEMICALS R&D CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com