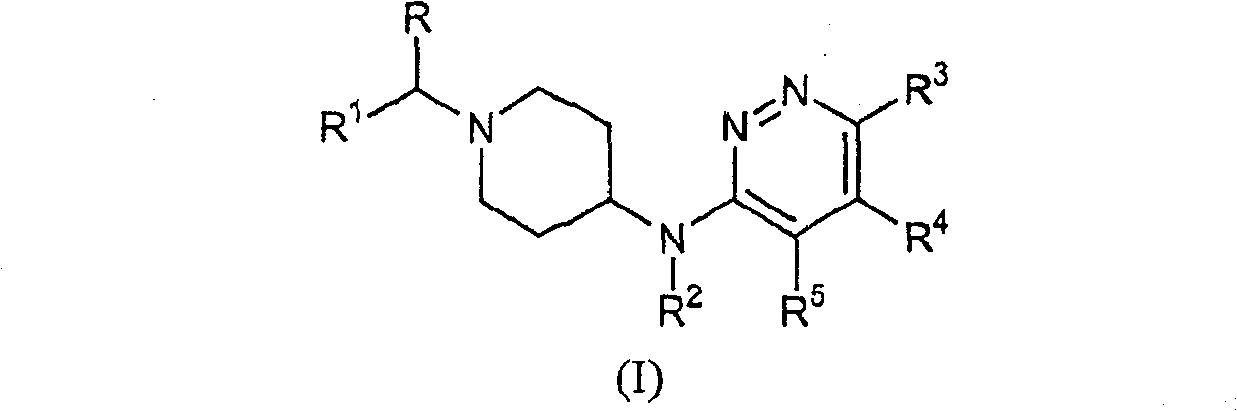
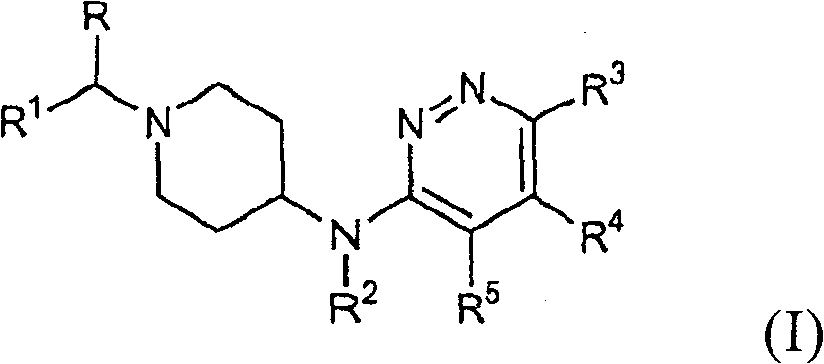
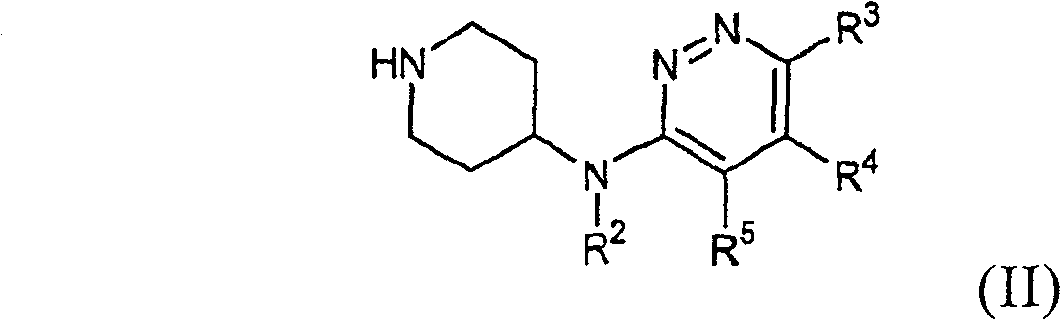
Piperidin-4-yl-pyridazin-3-ylamine derivatives as fast dissociating dopamine 2 receptor antagonists
A technology of pyridazine and piperidine is applied in the field of compounds that rapidly dissociate dopamine 2 receptor antagonists, and can solve the problems of lack of dopamine receptor blocker properties and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0111] N-[1-(3,4-difluorobenzyl)hexahydropyridin-4-yl]-6-(trifluoromethyl)pyridazin-3-amine (E1)
[0112]
[0113] 3,4-Difluorobenzaldehyde (1.52 g , 10.6 mmol) and sodium triacetoxyborohydride (2.24 g, 10.6 mmol). After stirring for 18 hours, the reaction mixture was quenched with 1N sodium hydroxide, the organic layer was removed, dried and the solvent was evaporated in vacuo. The crude product was purified by chromatography (silica; 2%-5% ammonia in methanol (7M) / dichloromethane) to afford E1 (2.39 g, 61%). C 17 h 17 f 5 N 4 The required value is 372; the measured value is 373 (MH + ); melting point: 167.7-168.9°C. 1 H NMR (DMSO-D6) δ1.50 (qd, J=11.5, 3.7Hz, 1H), 1.96 (br.d, J=12.4Hz, 2H), 2.12 (td, J=11.4, 2.6Hz, 2H) , 2.78(br.d, J=11.3Hz, 2H), 3.48(s, 2H), 3.90(br.s, 1H), 6.94(d, J=9.4Hz, 1H), 7.13-7.19(m, 1H ), 7.32-742 (m, 2H), 7.53 (br.d, J=7.3Hz, 1H), 7.63 (d, J=9.4Hz, 1H).
example 2
[0115] N-[1-(4-fluorobenzyl)hexahydropyridin-4-yl]-6-(trifluoromethyl)pyridazin-3-amine (E2)
[0116]
[0117] A mixture containing D2 (1.7 g, 5.32 mmol), 4-fluorobenzaldehyde (0.66 g, 5.32 mmol), di-isopropylethylamine (1.37 g, 10.6 mmol) and triacetoxy borohydride (On resin) (Aguno Technologies; 2.2 mmol / g; 3 equiv) in dichloroethane (10 mL) was stirred at room temperature for 18 hours. After this period, the reaction mixture was filtered and the solvent was evaporated in vacuo. The crude product was purified by chromatography (silica; 2%-6% ammonia in methanol (7M) / dichloromethane) to afford E2 (0.79 g, 42%). C 17 h 18 f 4 N 4 The required value is 354; the measured value is 355 (MH + ); melting point: 163.3-165.3°C. 1 H NMR (DMSO-D6) δ1.48(q, J=10.8Hz, 2H), 1.95(br.d, J=12.5Hz, 2H), 2.09(t, J=11.1Hz, 2H), 2.78(br .d, J=11.3Hz, 2H), 3.47(s, 2H), 3.89(br.s, 1H), 6.94(d, J=9.4Hz, 1H), 7.15(t, J=8.8Hz, 2H) , 7.34 (dd, J=8.3, 5.7Hz, 2H), 7.53 (br.d, J=7.3Hz, 1H), 7....
example 12
[0119] N-[1-(3,4-difluorobenzyl)hexahydropyridin-4-yl]-N-methyl-6-(trifluoromethyl)pyridazin-3-amine (E12)
[0120]
[0121] Containing D5 (480 mg, 2 mmol), 3-chloro-6-trifluoromethylpyridazine (182 mg, 1 mmol) and diisopropylethylamine (260 mg, 2 mmol) in normal - A solution in butanol (4 mL) was heated at 190° C. for 2 hours in a microwave reactor (Emrys Optimizer; 0-9 bar). After this period, the reaction mixture was evaporated in vacuo and the residue was extracted with dichloromethane. The organic layer was washed with saturated sodium bicarbonate solution, dried (MgSO 4 ) and the solvent was evaporated in vacuo. Purification was performed by reverse phase HPLC (conditions as described previously) to afford E12 (210 mg, 54%). C 18 h 19 f 5 N 4 The required value is 386; the measured value is 387 (MH + ). 1 H NMR (CDCl 3 )δ1.74(br.d, J=12.0Hz, 2H), 1.88(qd, J=12.0, 3.9Hz, 2H), 2.17(td, J=11.8, 2.5Hz, 2H), 2.96(br.d , J=11.6Hz, 2H), 3.00(s, 3H), 3.47(s, 2H), 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com