Formyl aniline substituted sulfhydryl pyrrolidine carbpenem compounds
A compound, methyl technology, applied in the field of matter, can solve the problems of not meeting clinical needs, weak antibacterial activity of MRSA, and low clinical availability.
Active Publication Date: 2010-11-17
XUANZHU BIOPHARMACEUTICAL CO LTD
View PDF3 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Due to the continuous increase of bacterial resistance and the limitation of digestive tract absorption due to the abuse of antibiotics, the carbapenems currently on the market can only be administered as injections in clinical practice, and the clinical utilization is not high, and the antibacterial activity against MRSA Weak, can no longer meet clinical needs
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
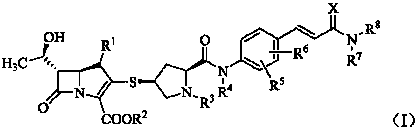
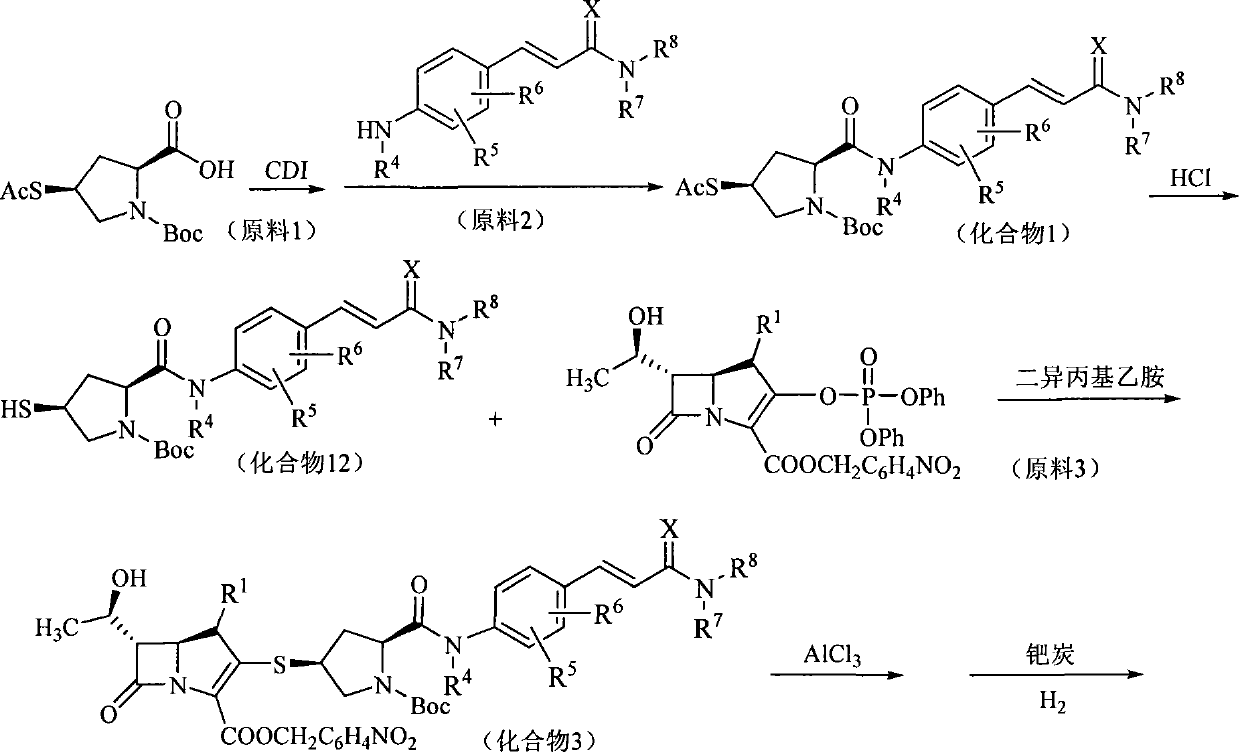
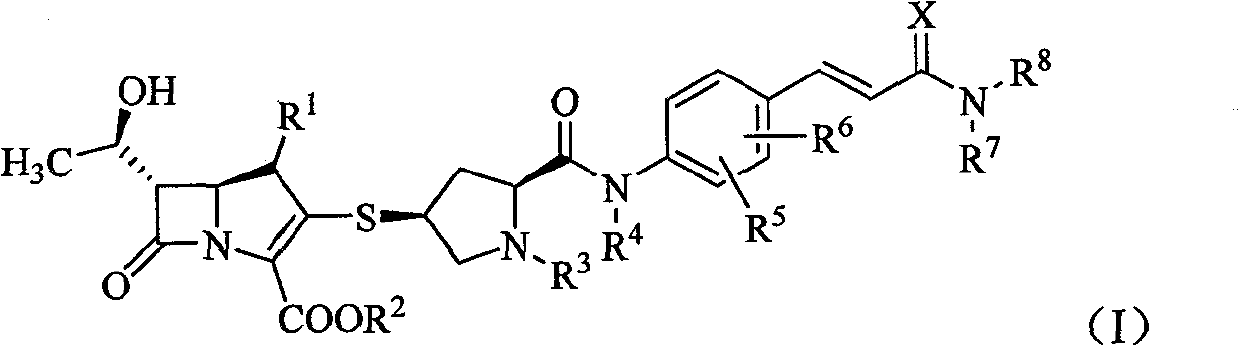
The present invention relates to a formanilide substituted sulfhydryl pyrrolidine carbapenems compound as shown in Formula (I), easily hydrolyzed ester thereof, acceptable non-toxicity salt thereof in pharmacy, isomer thereof, hydrate thereof, and hydrate of ester or salt thereof. The present invention also relates to a preparation method of the compound as shown in Formula (I), application of the compounds as active substances for medicine, in particular to the application for preparing the medicine used for treating and / or preventing infectious diseases. The R<1>, R<2>, R<3>, R<4>, R<5>, R<6>, R<7>, R<8> and X are defined in details in the specification.
Description
1. Technical field The present invention relates to mercaptopyrrolidine carbapenems substituted by formanilide, their easily hydrolyzed esters, their pharmaceutically acceptable non-toxic salts, their isomers, their hydrates, and the hydration of their esters or salts Compounds, preparation methods of these compounds, pharmaceutical compositions containing these compounds, and the use of these compounds for the preparation of drugs for treating and / or preventing infectious diseases belong to the field of medical technology. 2. Background technology Carbapenems are new broad-spectrum, enzyme-resistant and highly effective β-lactam antibiotics developed in the 1970s. In 1976, Thiamycin, the first carbapenem antibiotic, was discovered, but it was not used clinically due to its poor chemical stability. Later, the chemical structure modification of thiamycin produced a series of carbapenem derivatives. At present, such drugs that have been marketed include imipenem, panipenem, ...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): A61P31/04C07D477/20A61K31/407
CPCY02P20/55
Inventor 黄振华
Owner XUANZHU BIOPHARMACEUTICAL CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com