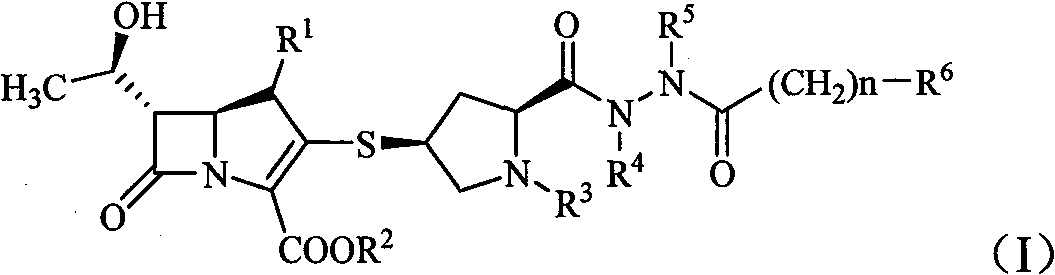
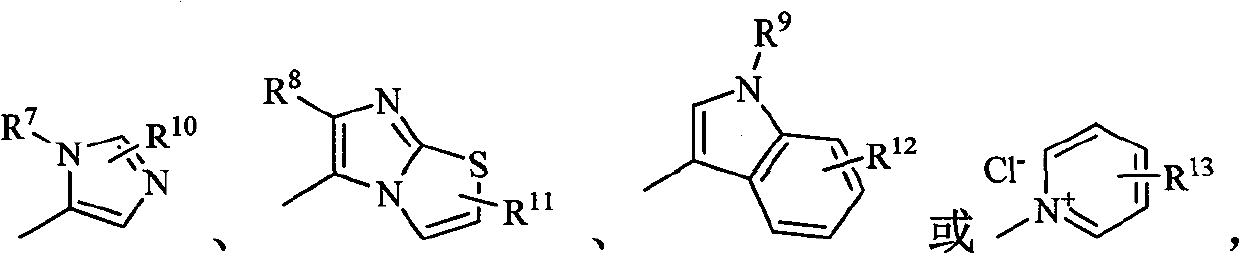
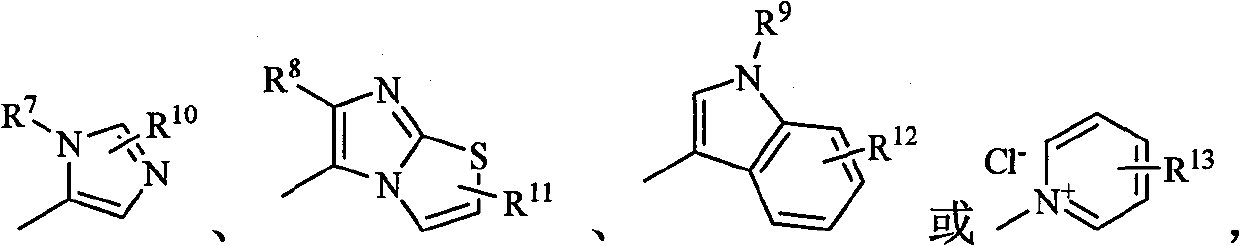
Carbapenem compound containing formyl hydrazino
A compound, carbamoyl technology, applied in the field of carbapenem compounds containing carboxyl hydrazide group, can solve the problems of inability to meet clinical needs, weak antibacterial activity of MRSA, low clinical availability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105] Example 1 Preparation of (2S, 4S)-4-mercapto-2-formyl (1-methyl-1H-imidazolyl-5-formyl)hydrazine-1-(tert-butoxycarbonyl)pyrrolidine
[0106] 14.5 g (50 mmol) of (2S,4S)-4-acetylthio-2-carboxy-1-(tert-butoxycarbonyl)pyrrolidine and 150 ml of anhydrous tetrahydrofuran were added to a dry reaction flask. Under the protection of nitrogen, 13g (80mmol) of 1,1-carbonyldiimidazole was added at room temperature, reacted for 0.5h, and 8.4g (60mmol) of 1-methyl-1H-imidazolyl-5-formylhydrazine was added below 0°C. Tetrahydrofuran solution was 100ml, and the reaction was continued for 0.5h. Then 60ml of 1mol / L hydrochloric acid was added dropwise, extracted with ethyl acetate (100ml×2), the organic phase was washed with water and saturated sodium chloride solution successively, concentrated under reduced pressure, the residue was added with 150ml of 3mol / L hydrochloric acid, stirred for 2h, and The dilute alkaline solution was adjusted to be basic, and a solid was precipitated, ...
Embodiment 2
[0107] Example 2 (4R, 5S, 6S)-3-[(2S, 4S)-2-formyl (1-methyl-1H-imidazolyl-5-formyl)hydrazine-1-(tert-butoxycarbonyl )pyrrolidin-4-yl]thio-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo-[3,2,0]hept-2 Preparation of -ene-2-carboxylic acid p-nitrobenzyl ester
[0108]In a dry reaction flask, add (4R, 5S, 6S)-3-diphenoxyphosphoryloxy-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1- Azabicyclo-[3,2,0]hept-2-ene-2-carboxylic acid p-nitrobenzyl ester 11.9g (20mmol) in 120ml of acetonitrile solution, cooled to below -10°C, add diisopropylethylamine 5ml and (2S,4S)-4-mercapto-2-formyl (1-methyl-1H-imidazolyl-5-formyl)hydrazino-1-(tert-butoxycarbonyl)pyrrolidine 8.1g (22mmol) 80ml of acetonitrile solution, stirred at 0°C for 15h. After the reaction was completed, 300 ml of ethyl acetate was added to dilute, washed with water and saturated brine successively, the organic layer was dried and concentrated to obtain 10.1 g of solid, yield: 71.2%.
Embodiment 3
[0109] Example 3 (4R, 5S, 6S)-3-[(2S, 4S)-2-formyl (1-methyl-1H-imidazolyl-5-formyl)hydrazine Base-pyrrolidin-4-yl]thio-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo-[3,2,0]heptyl- Preparation of 2-ene-2-carboxylic acid (compound A)
[0110] (4R, 5S, 6S)-3-[(2S, 4S)-2-formyl(1-methyl-1H-imidazolyl-5-formyl)hydrazino-1-(tert-butoxycarbonyl)pyrrole Alk-4-yl]thio-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo-[3,2,0]hept-2-ene 7.1 g (10 mmol) of p-nitrobenzyl 2-carboxylate was dissolved in 50 ml of dichloromethane, 10 ml of anisole and 20 ml of nitromethane were added, and 1 mol / L of aluminum trichloride was added dropwise at -50°C Nitromethane solution 100ml, stir at -40°C for 2h, add 200ml of water, precipitate solid, filter, dissolve the filter cake in a mixture of 400ml of THF and 30ml of water, add 2g of 10% palladium-carbon, stir at room temperature under 5MPa hydrogen pressure After reacting for 2 hours, palladium carbon was filtered off, 150 ml of THF was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com