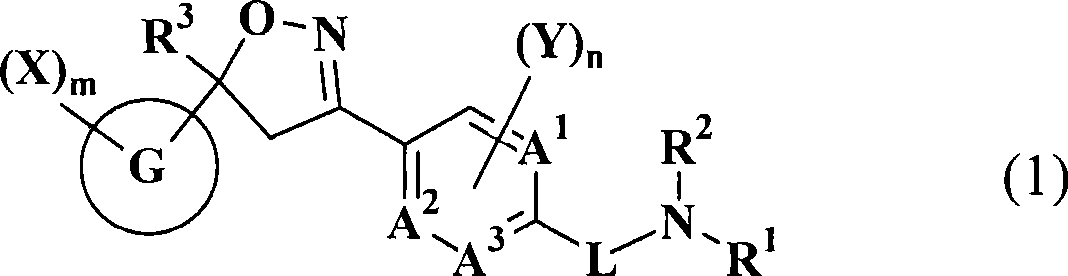
Substituted isoxazoline compound and pest control agent
A compound, isoxazoline technology, applied in plant growth regulators, biocides, animal repellents, etc., can solve problems such as unknown usefulness of pest control agents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[5764] Hereinafter, as examples, the synthesis examples and test examples of the present invention will be described in more detail, but the present invention is not limited to these.
Synthetic example 1
[5767] Preparation of the compound of the present invention using L-COS (a parallel liquid phase synthesis device manufactured by MORITEX).
[5768] Step 1: Preparation of 3,5-dichloro-1-(1-trifluoromethylvinyl)benzene
[5769] In a solution of 3,5-dichlorophenylboronic acid 25.0g in 200ml tetrahydrofuran and 100ml water, add 27.5g 2-bromo-3,3,3-trifluoropropene, 38.0g potassium carbonate and 1.84g dichlorobis(three Phenylphosphine) palladium(II) was stirred under heating and reflux for 3 hours. After the reaction is completed, let it cool to room temperature, add 500ml ice water, and extract with ethyl acetate (500ml×1). The organic layer was washed with water, then dried with anhydrous sodium sulfate, the solvent was distilled off under reduced pressure, and the residue was purified by silica gel column chromatography in which the residue was eluted with hexane to obtain 25.7 g of the colorless oily target product.
[5770] 1 H NMR(CDCl 3 , Me 4 Si, 300MHz) δ7.41(t, J=2.0Hz, 1H)...
Synthetic example 2
[5806] 1-[4-[5-(3,5-Dichlorophenyl)-5-trifluoromethyl-4,5-dihydroisoxazol-3-yl]phenylmethyl]-3-(2 , 2,2-Trifluoroethyl)urea (Compound No. 5-001 of the present invention).
[5807] 0.20 g of 2,2,2-trifluoroethylamine was added to a 7.0 mL solution of 0.32 g of 1,1'-carbonyldi-1H-imidazole in tetrahydrofuran, and the mixture was stirred at room temperature for 1.5 hours. Next, 0.39 g of 3-[4-(aminomethyl)phenyl]-5-(3,5-dichlorophenyl)-5- synthesized in Step 6 of Synthesis Example 1 was added to the reaction mixture. A 5.0 mL solution of trifluoromethyl-4,5-dihydroisoxazole in tetrahydrofuran was stirred at room temperature for 2.5 hours. After the completion of the reaction, the reaction mixture was diluted with 20 mL of ethyl acetate, washed with water (15 mL×2), and then dehydrated and dried with saturated brine and anhydrous magnesium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by medium-pressure preparative liquid chromatography (Y...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com