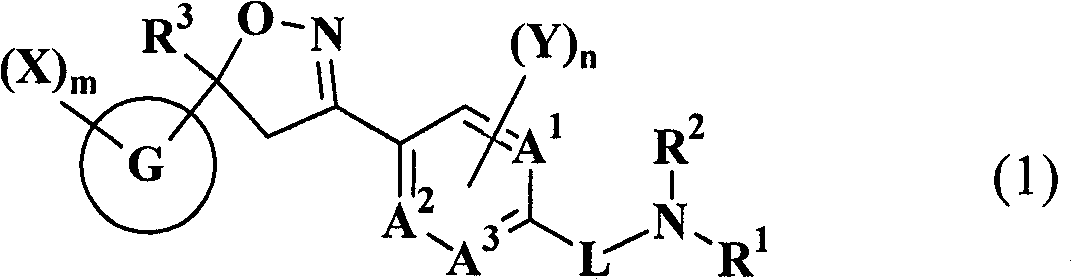
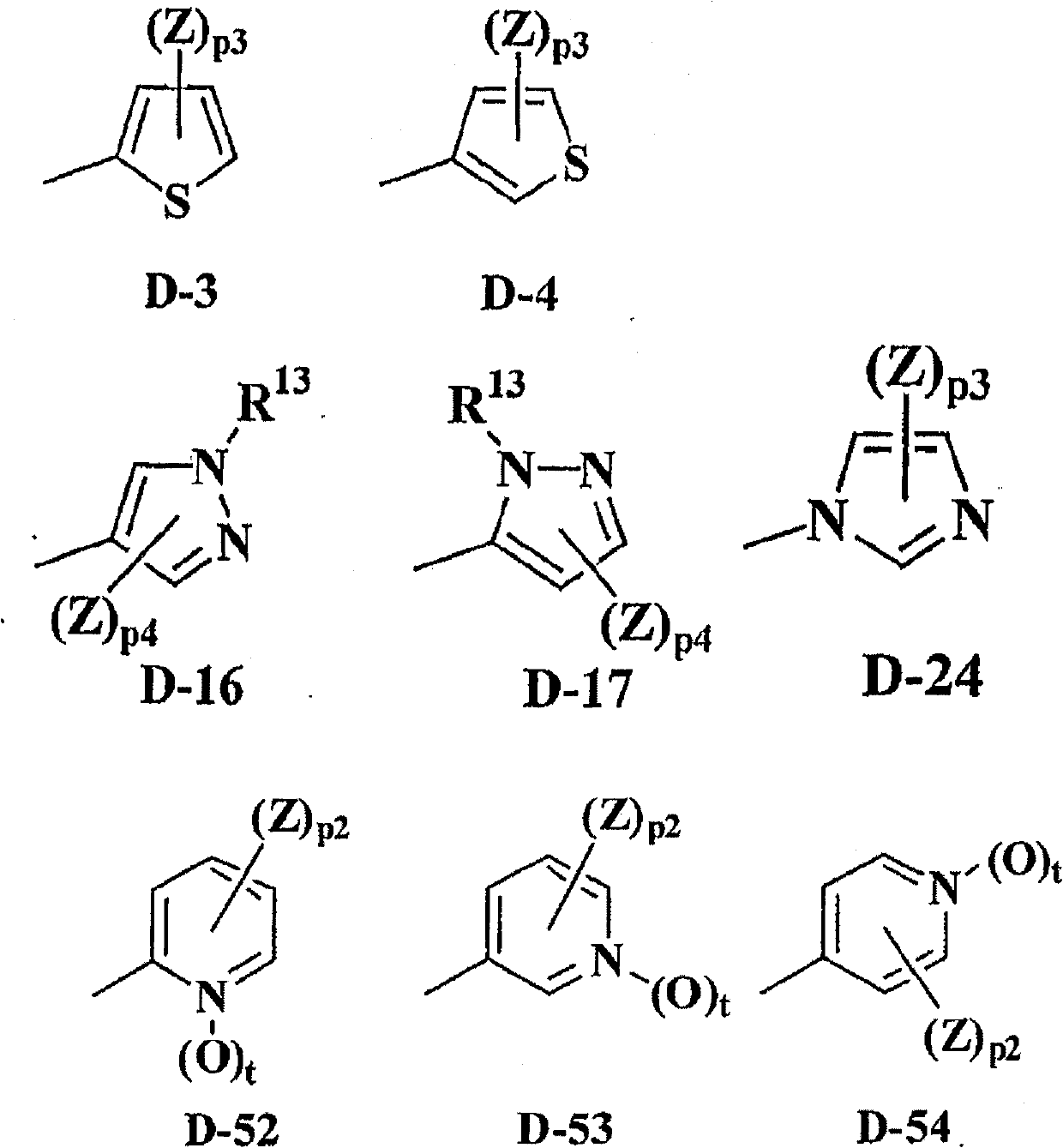
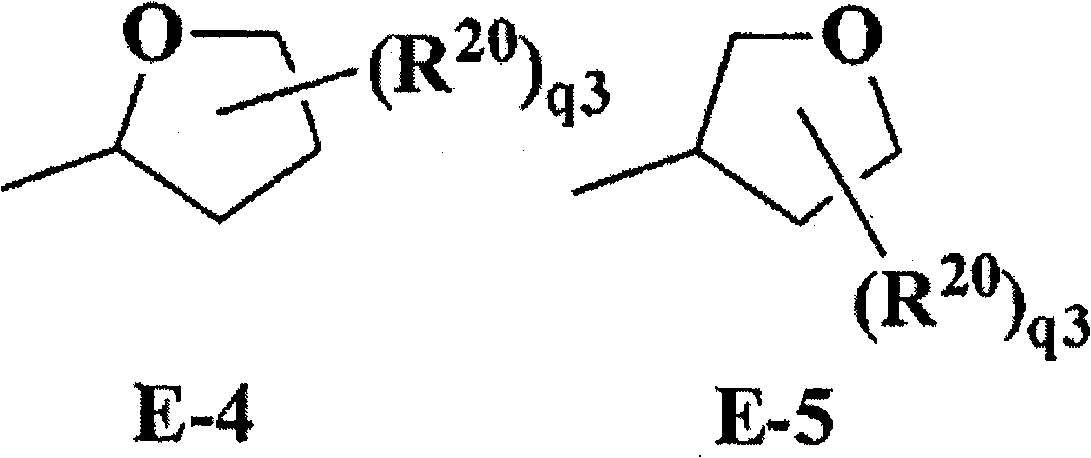
Substituted isoxazoline compound and pest control agent
An isoxazoline and compound technology, which is applied in the field of pest control agents and can solve problems such as unknown usefulness of pest control agents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0785] [synthesis example]
Synthetic example 1
[0788] Step 1: Preparation of 3,5-dichloro-1-(1-trifluoromethylvinyl)benzene
[0789] In a solution of 25.0 g of 3,5-dichlorophenylboronic acid in 200 ml of tetrahydrofuran and 100 ml of water, add 27.5 g of 2-bromo-3,3,3-trifluoropropene, 38.0 g of potassium carbonate and 1.84 g of dichlorobis(trifluoropropene) Phenylphosphine) palladium (II), stirred under reflux for 3 hours. After the reaction was complete, let cool to room temperature, add 500ml of ice water, and extract with ethyl acetate (500ml×1). The organic layer was washed with water, dried over anhydrous sodium sulfate, the solvent was distilled off under reduced pressure, and the residue was purified by silica gel column chromatography eluting the residue with hexane to obtain 25.7 g of the target product as a colorless oil.
[0790] 1 H NMR (CDCl 3 , Me 4 Si, 300MHz) δ7.41(t, J=2.0Hz, 1H), 7.3-7.35(m, 2H), 6.05(q, J=3.2Hz, 1H), 5.82(q, J=3.2Hz, 1H) .
[0791] Step 2: Preparation of methyl 4-[5-(3,5-dichlorop...
Synthetic example 2
[0827] 0.20 g of 2,2,2-trifluoroethylamine was added to a solution of 0.32 g of 1,1,-carbonyldi-1H-imidazole in 7.0 mL of tetrahydrofuran, and the mixture was stirred at room temperature for 1.5 hours. Next, to this reaction mixture was added 0.39 g of 3-[4-(aminomethyl)phenyl]-5-(3,5-dichlorophenyl)-5- A 5.0 mL solution of trifluoromethyl-4,5-dihydroisoxazole in tetrahydrofuran was stirred at room temperature for a further 2.5 hours. After completion of the reaction, the reaction mixture was diluted with 20 mL of ethyl acetate, washed with water (15 mL×2), dehydrated and dried using saturated brine and anhydrous magnesium sulfate in sequence, and the solvent was distilled off under reduced pressure. The residue was purified by medium-pressure preparative liquid chromatography (YFLC-Wprep, Yamazen Co., Ltd.) eluting with ethyl acetate-hexane (gradient 1:8 to 1:1) to obtain 0.43 g of the desired product as a resinous mass.
[0828] 1 H NMR (CDCl 3 , Me 4Si, 300MHz) δ7.63(d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com