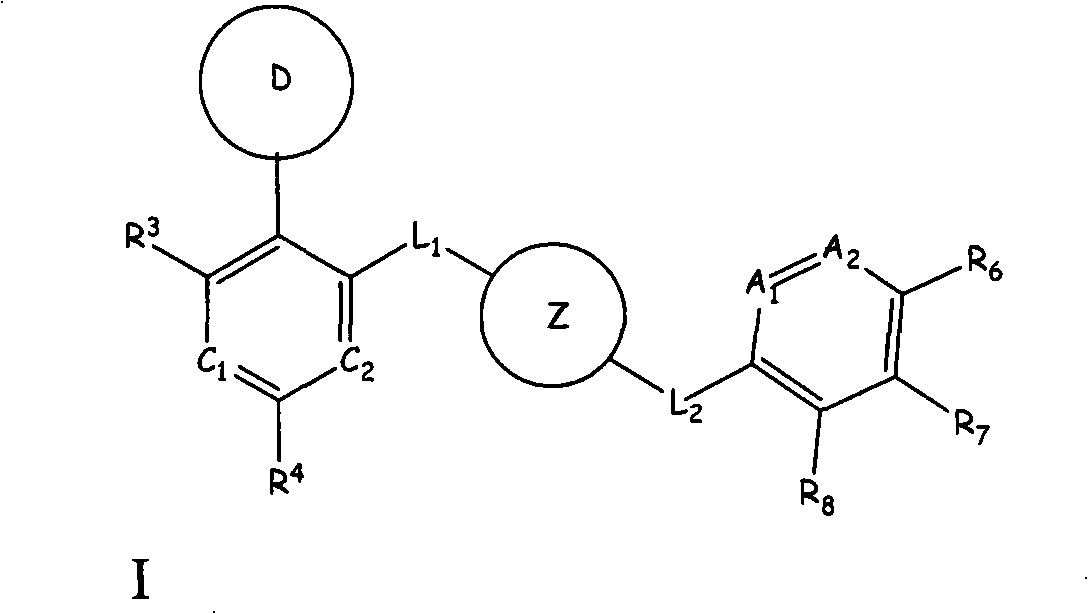
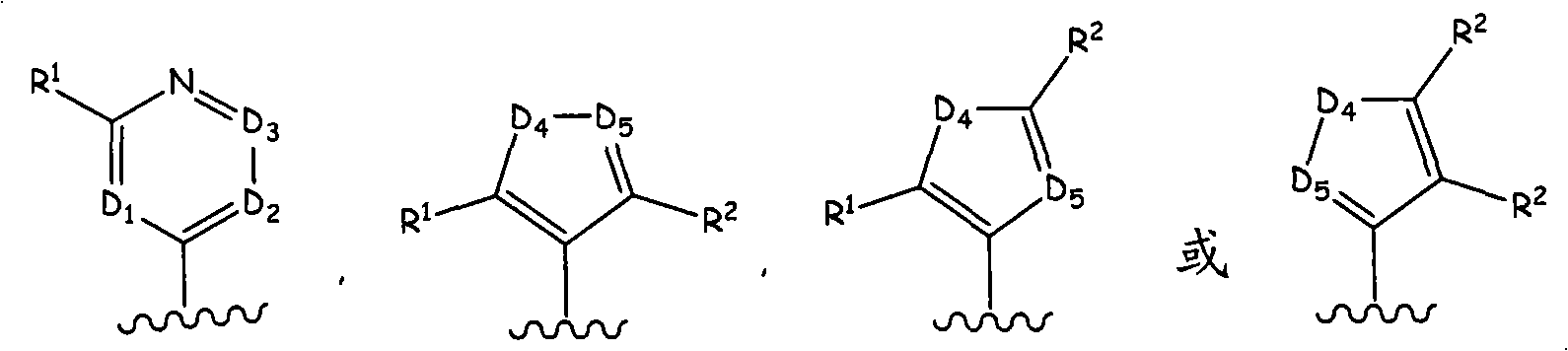
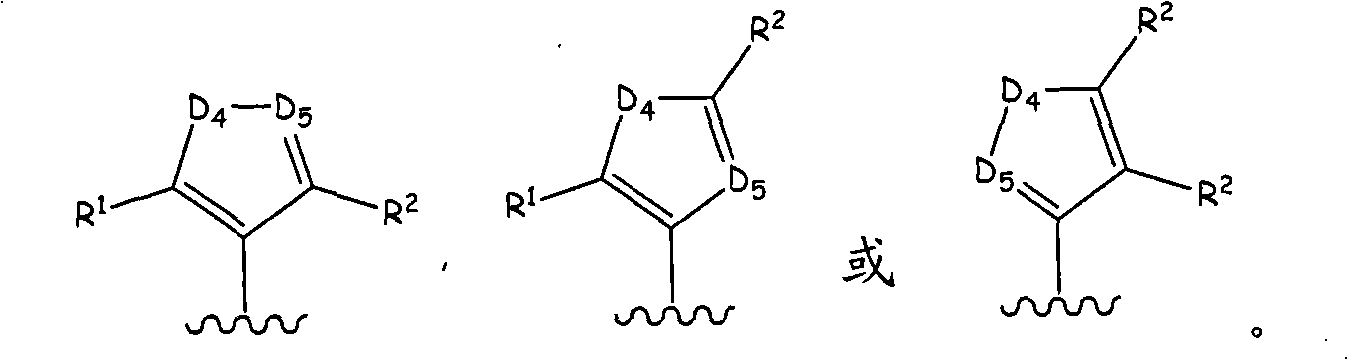
Aurora kinase modulators and method of use
A solvate, stereoisomeric technology for compounds and compositions that modulate Aurora kinases, control cell proliferation and treat cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0366]
[0367] Synthesis of 2-chloro-4-(2-chloro-pyridin-3-yl)-[1,3,5]triazine
[0368] Preparation of step 1.2-chloro-nicotinamide
[0369] 2-Chloro-3-cyanopyridine (5.0 g, 36 mmol) was dissolved in anhydrous EtOH (100 mL) at 0 °C. HCl was bubbled through the mixture for 3 h, the mixture was sealed and centrifuged (ca. 8° C.) overnight. After concentration, the residue was stirred with ammonium acetate (5.5 g) in 100 mL of IpOH. After 12h, with concentrated NH 4 OH solution, adjust the pH to 9 (from 4), and continue stirring for 2 more days. The mixture was concentrated and subjected to flash chromatography (10:1:0.1 CH 2 Cl 2 / MeOH / NH 4 OH) purification. Trituration in hot tBuOMe / IpOH removed some residual amide by-product to give the product as a white solid.
[0370] Step 2: Amino-(2-chloro-pyridin-3-yl)-methylcyanamide
[0371] 2-Chloro-nicotinamidine was suspended in 10 mL of IpOH with 500 mg of solid cyanamide, and the stirred solid was passed through t...
Embodiment 2
[0375]
[0376] Synthesis of [4-(2-chloro-pyridin-3-yl)-[1,3,5]triazin-2-yl]-methyl-amino
[0377] To a solution of 2-chloro-4-(2-chloro-pyridin-3-yl)-[1,3,5]triazine (10.0 g, 44.0 mmol) in 55 ml of dichloromethane at 0°C was added methylamine (45ml, 88.0mmol) in 2.0M THF. After stirring at room temperature for 18 h, the mixture was diluted with acetone, filtered through a pad of silica gel, and concentrated to give the desired product. MS m / z=222[M+H] + . C 9 h 8 ClN 5 Calculated value: 221.65.
Embodiment 3
[0379]
[0380] Synthesis of 4-(2-chloro-pyridin-3-yl)-pyrimidine
[0381] Step 1. Preparation of 1-(2-chloro-pyridin-3-yl)-3-dimethylamino-propenone
[0382] In a drying tube at 85°C, 1-(2-chloro-pyridin-3-yl)-ethanone (21.7g, 139mmol) was dissolved in 46mL N,N-dimethylformamide dimethylacetal (42g, 350mmol ) for 1.5h, then concentrated. The residue was purified by suction filtration chromatography (using 150 g of silica gel in a Buchner funnel, rapidly collecting fractions with 10:1, then 5:1 CH 2 Cl 2 / IpOH) to give the product as a yellow solid. MS m / z=211[M+H] + . C 10 h 11 ClN 2 O calculated value: 210.66.
[0383] Step 2. Preparation of 4-(2-chloro-pyridin-3-yl)-pyrimidine
[0384] Using a 500mL IpOH bath at room temperature as a cold source, at room temperature, N 2 Next, by intermittently adding small pieces of sodium metal (8.3 g, 360 mmol in total) to 400 mL of anhydrous methanol, sodium methoxide was generated over 1.5 hours. Formamidine acetate ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com