Treating obesity with muscarinic receptor m1 antagonists
An antagonist, obesity technology, applied in the direction of application, biocide, animal repellent, etc., can solve the problems that are not disclosed or implied, the ability to hinder the sensitivity of lipogenesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
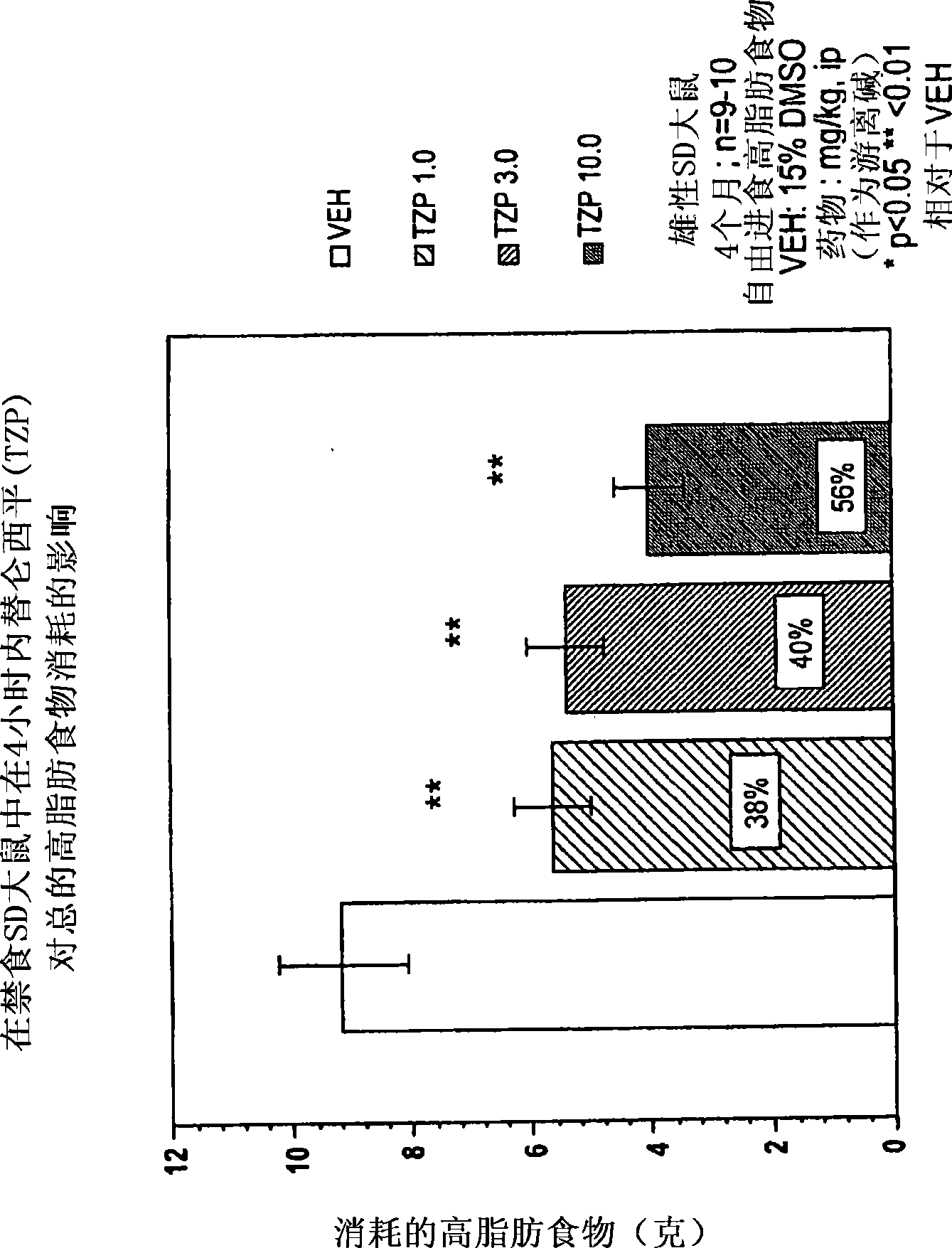
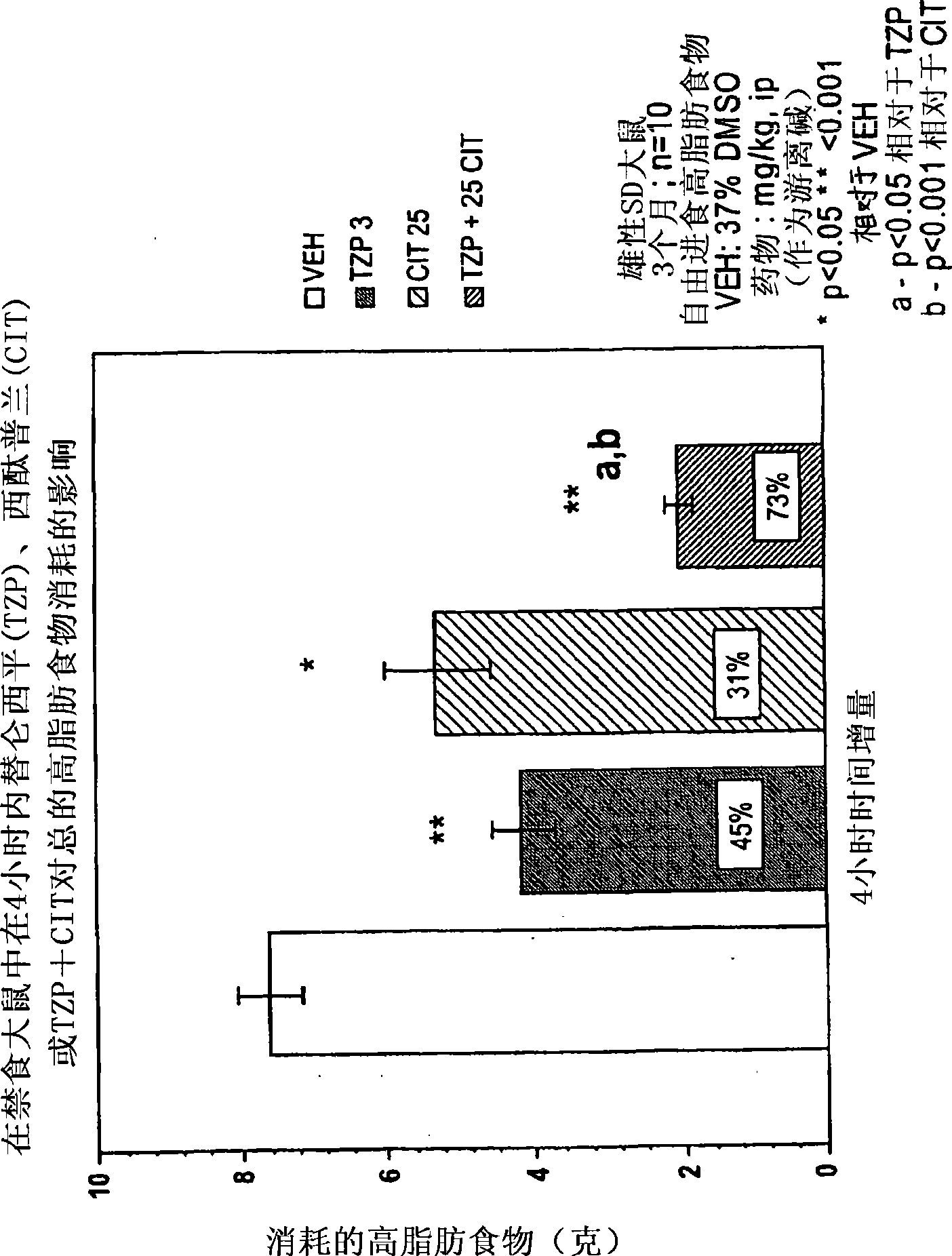
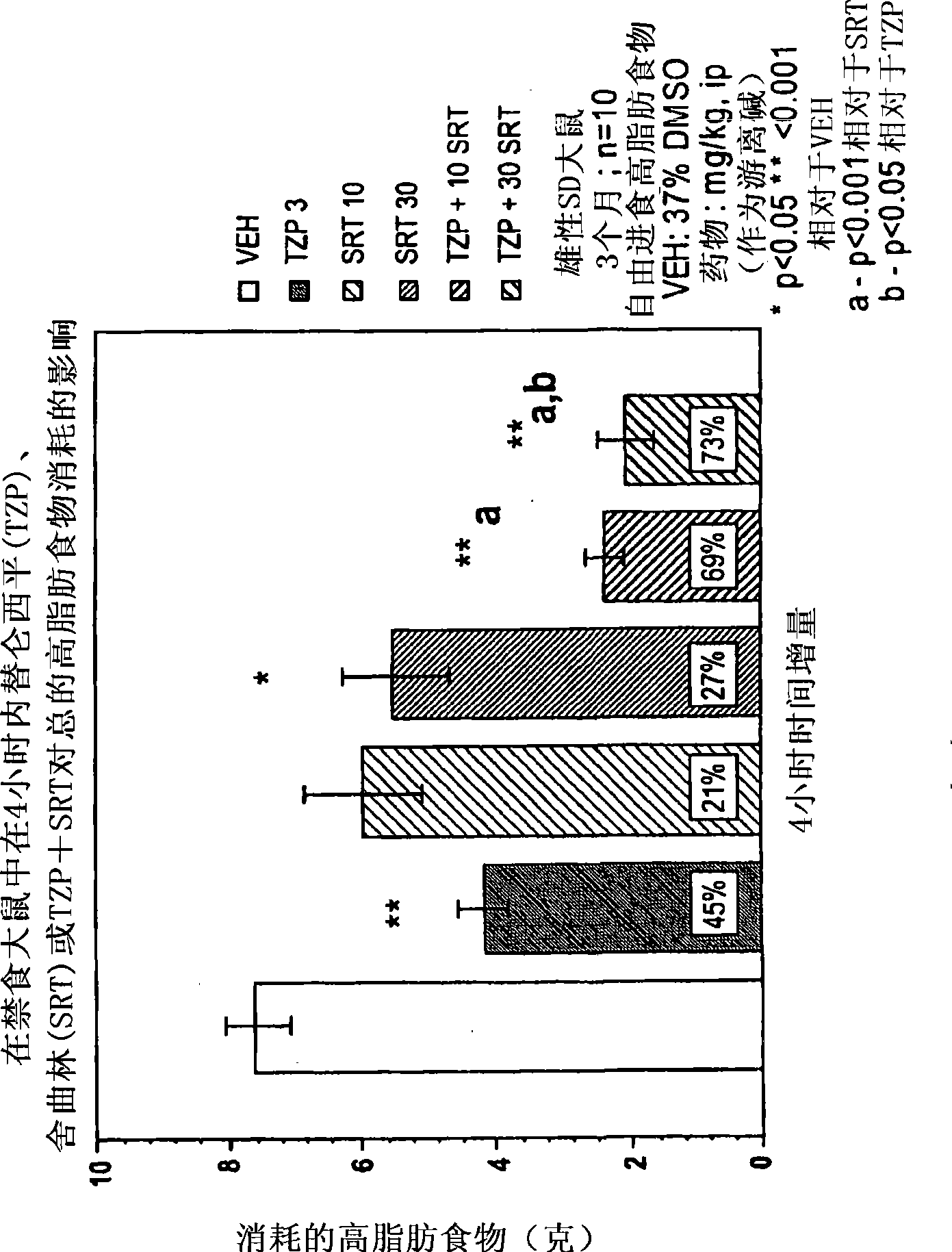
[0179] Appetite Suppressant: Three month old (300-350 g) male Sprague-Dawley rats (housed individually) were used to evaluate the appetite suppressant effect of the compounds. Rats were acclimated to a "high fat" diet (BioServ Diet #F3282 or Research Diets #12451) for two weeks prior to testing (free access to food and water). The day before the experiment (at 5:00 PM), food was removed from the cage to stimulate feeding when it was returned the next morning (water supply was maintained throughout the experiment). Rats (n=8-10 / dose group) were dosed intraperitoneally (ip) or orally (po) the compound under investigation before food was provided, returned to their cages and immediately given pre-weighed food. Four hours after dosing, food was removed from cages, weighed, recorded (4 hour consumption) and returned to cages until the next morning. Twenty-four hours after dosing, the food remaining was weighed again and recorded (24-hour consumption). Cumulative consumption (expr...
Embodiment 2
[0192] Reduction of Body Weight Gain: Appetite Suppression: Three to four month old (475-550 grams) male Sprague-Dawley rats (housed individually) were used to evaluate the ability of compounds to prevent body weight gain. At the beginning of the chronic experiment, rats had been maintained (free access) on a "high fat" diet (BioServ Diet #F3282 or Research Diets #12451 ) for approximately one month. Individual body weight and water intake were recorded three times per week throughout the duration of the experiment. Two weeks after data collection, rats were weight balanced to produce test groups with equal mean body weights. Under isoflurane-induced anesthesia, rats were surgically implanted (subscapularis, subcutaneous [sc] placement) with mini-osmotic pumps (Alzet2ML2) containing appropriate drug concentrations (based on average body weight and calculated delivery duration). Alternatively, for studies using the oral route of administration, rats were dosed by daily gavage ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com