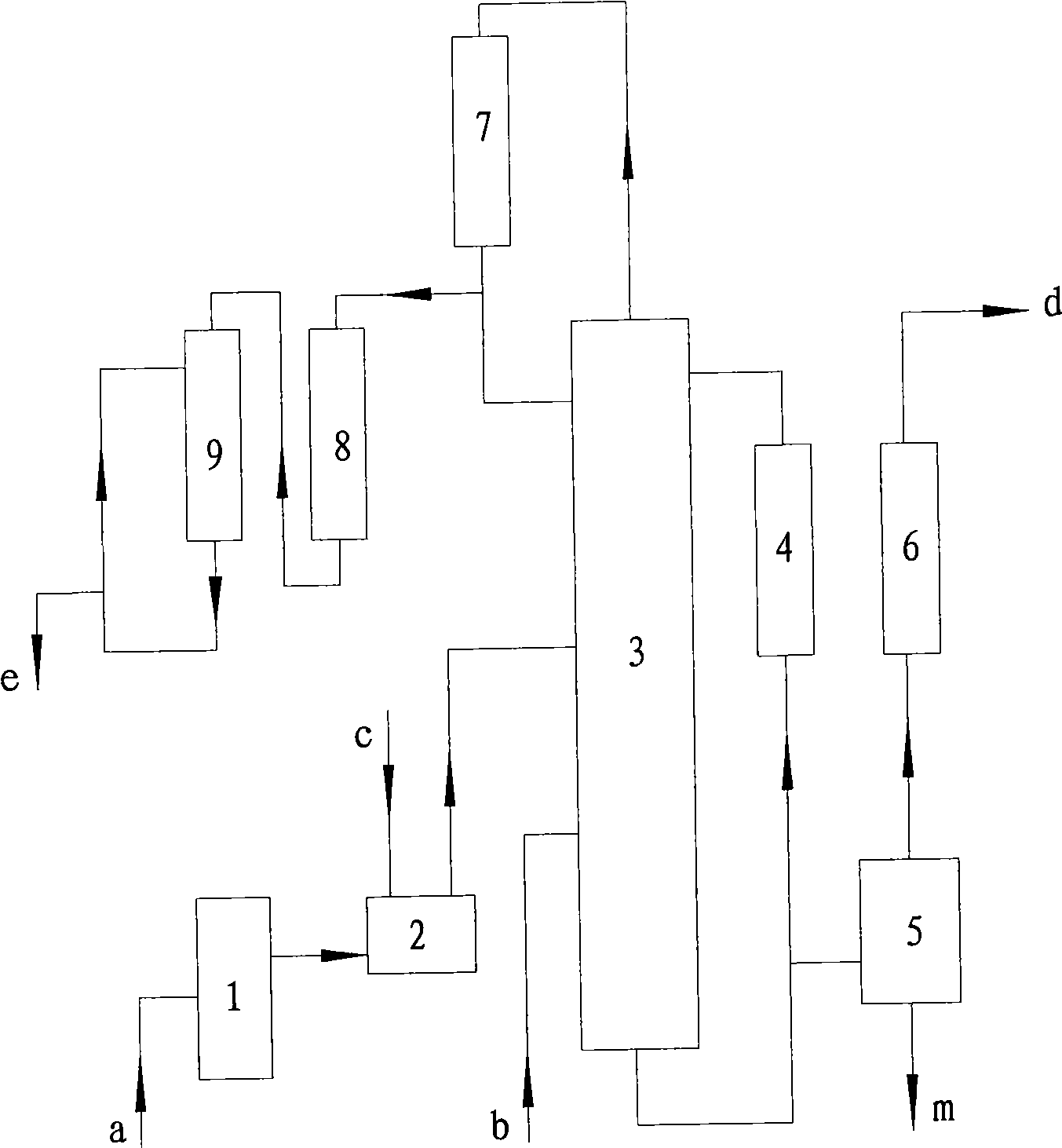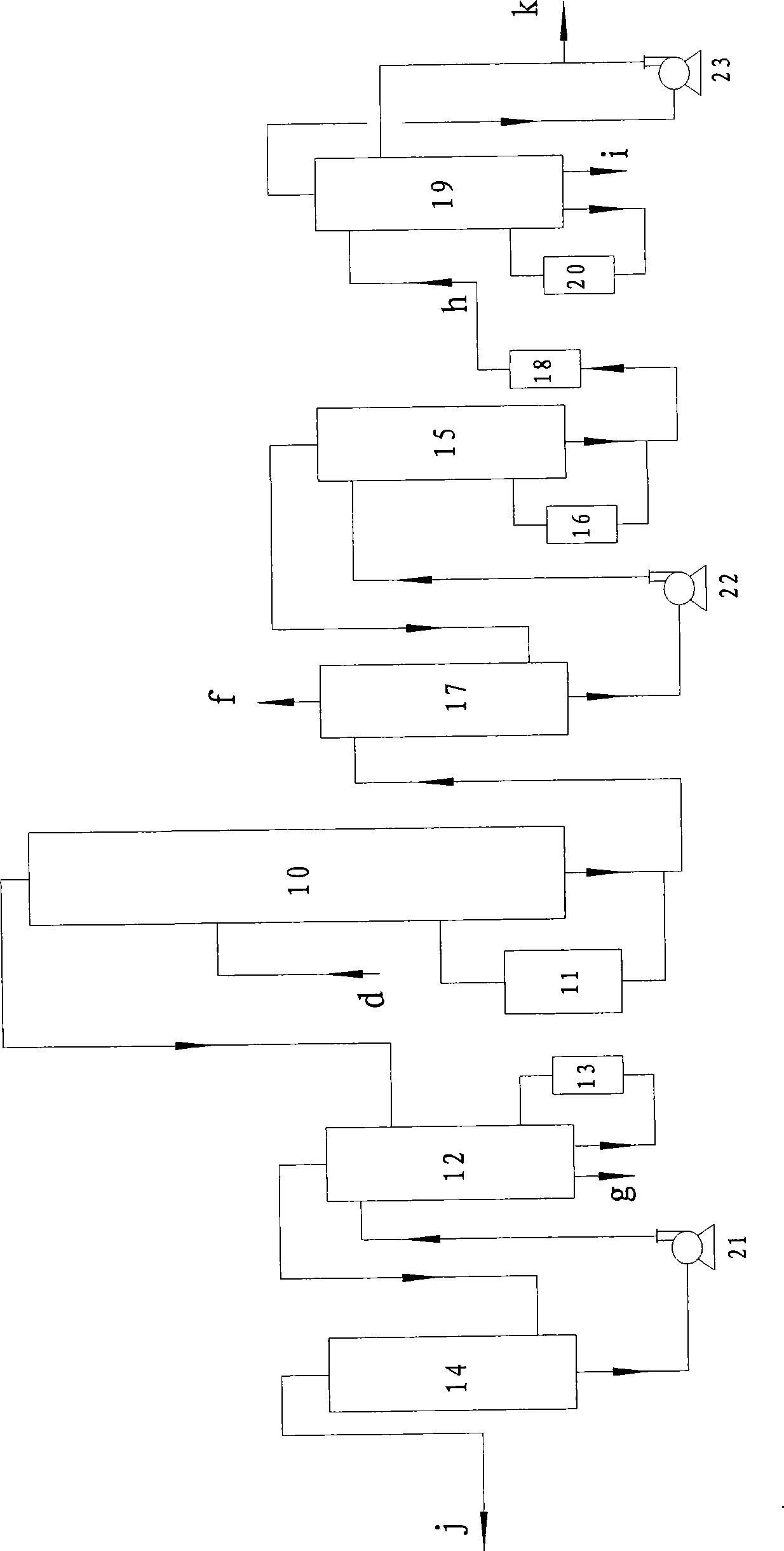Method for preparing p-chlorotoluene and o-chlorotoluene by chlorination toluene
A technology of p-chlorotoluene and o-chlorotoluene, applied in chemical instruments and methods, preparation of halogenated hydrocarbons, organic chemistry, etc., can solve problems such as environmental pollution, achieve the effects of saving investment, facilitating maintenance, and reducing operating costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
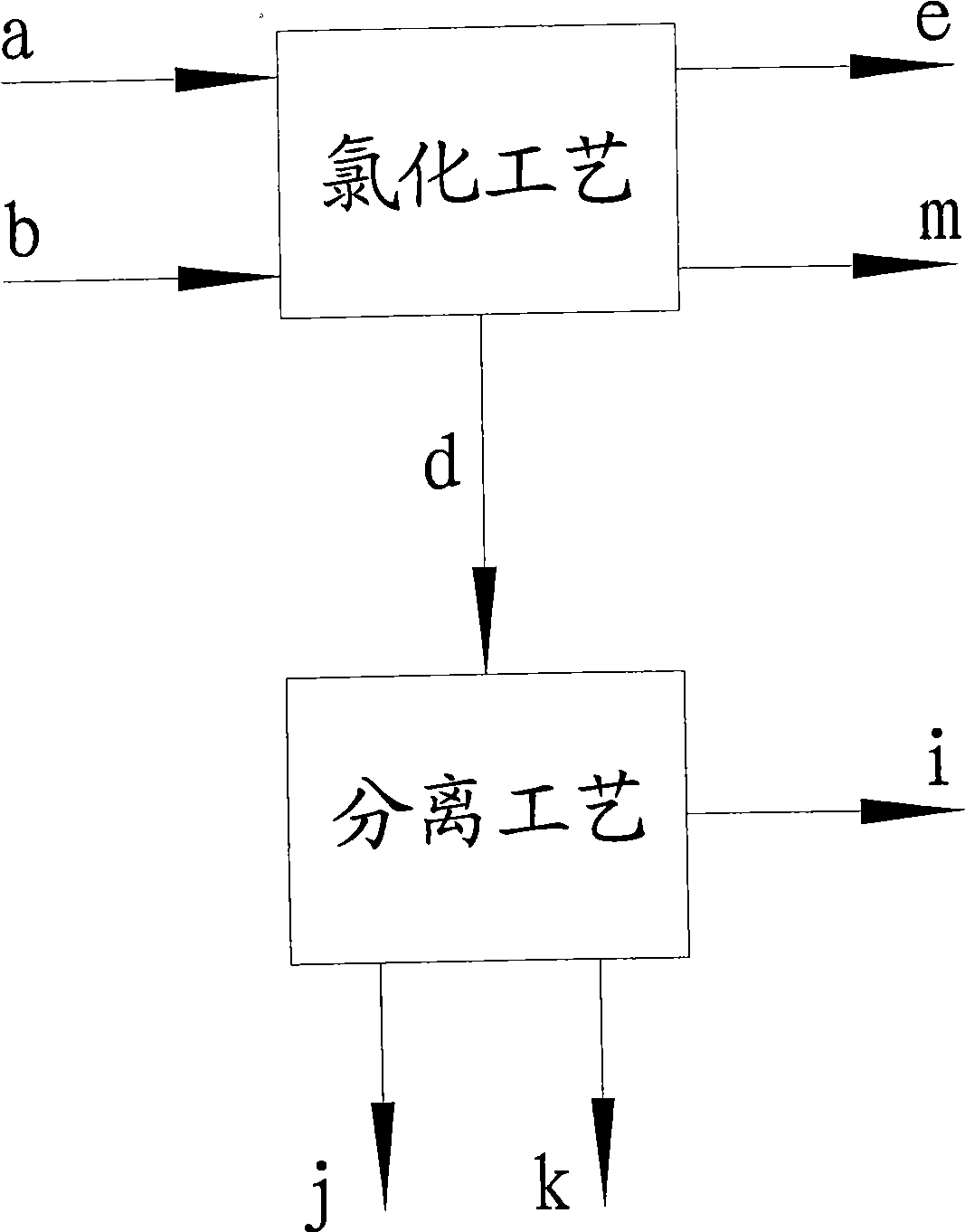
Image
Examples
Example Embodiment
[0030] Example 1:
[0031] Toluene a: 7.6 tons, dried by dryer 1 at a flow rate of 2.5 tons / hour, and then flowed into batching tank 2, adding 1000g of co-catalyst sulfur, fully stirring and pumping into chlorination tower 3, and chlorine gas flowing into chlorination at a flow rate of 0.9 tons / hour Tower 3, cyclic chlorination reaction, the reaction mole of chlorine and toluene is 1:1, the reaction temperature is controlled at 67℃, the absolute pressure in the chlorination tower 3 is 0.07Mpa, the main component of the catalyst is ferric chloride, and the auxiliary catalyst includes Sulfur or sulfide (S 2Cl 2 ), ferric trichloride is formed by the reaction of chlorine and iron ring in the chlorination process. The chlorination tower 3 is a fixed-bed tower reactor with a catalyst iron ring stacked in the lower part of the tower. Chlorine gas enters the reactor from six different positions in the lower part of the tower. The chlorination liquid is cyclically chlorinated, and the out...
Example Embodiment
[0036] Example 2:
[0037] Toluene a 9.5 tons, dried by dryer 1 at a flow rate of 2.0 tons / hour, flow into the batching tank 2, add 2000g of co-catalyst sulfur, fully stir and pump into the chlorination tower 3, and chlorine gas at a flow rate of 0.5 tons / hour into the chlorination tower 3. Cyclic chlorination reaction, the reaction mole of chlorine b and toluene a is 0.8:1, the reaction temperature is controlled at 32°C, and the internal pressure of the chlorination tower 3 is 0.06Mpa (absolute pressure). The content of the chlorinated solution is checked regularly, and the chlorination reaction is stopped when the index is reached. The hydrogen chloride gas passes through the absorption device to obtain 9.39 tons of hydrochloric acid with a concentration of 31.5%wt. The chlorinated solution is distilled and detoluene to obtain 10.22 tons of mixed chlorinated toluene, and 0.08 tons of residue is removed. 2.0 tons of toluene obtained from detoluene will be recycled for next time. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com