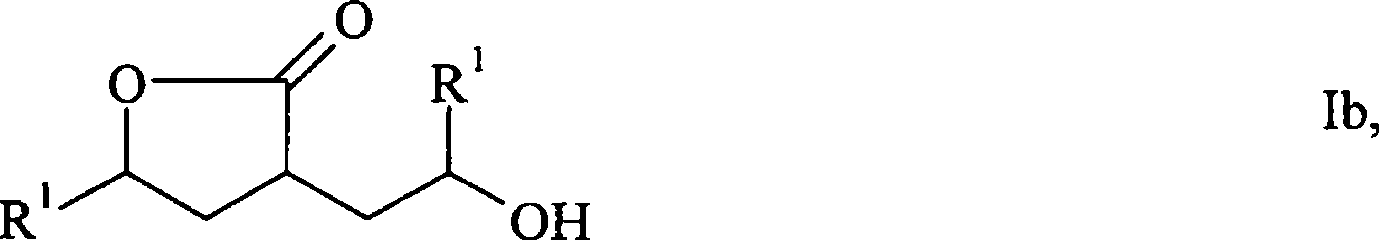
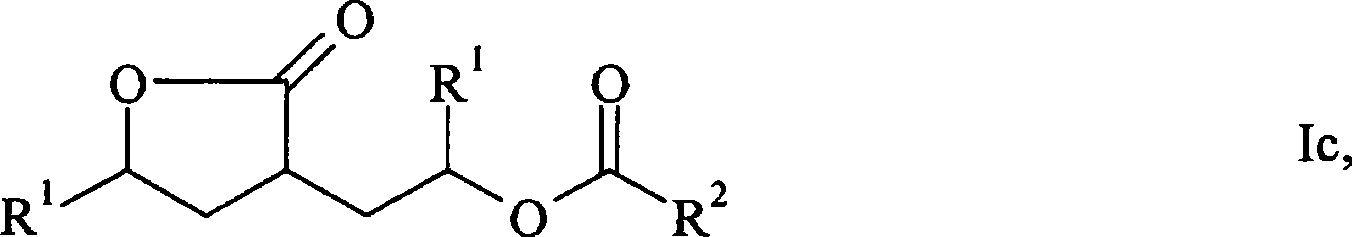
Process for the preparation of gamma-butyrolactones
A kind of alkyl, selected technology, applied in the field of preparation of γ-butyrolactone, can solve problems such as long time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Methyl acetoacetate ( MAA, 46.7 g, 0.40 mol) in methanol (MeOH, 144.0 g). The reaction mixture was heated to 40° C. within 0.5 hours and maintained at this temperature for 4.5 hours. Quantitative GC analysis showed that 53.7% of MAA was converted to α-acetylbutyrolactone (ABL) with a selectivity of 75.2%.
Embodiment 2
[0079] A solution of MAA (46.9 g 0.40 mol) in MeOH (144.0 g) was treated sequentially with EO (25.0 g, 1.4 eq.) and triethylamine (TEA, 40.7 g, 1.0 eq.) at 5°C. The reaction mixture was heated to 60° C. within 0.5 hours and maintained at this temperature for 2.5 hours. Quantitative GC analysis showed that 50.8% of MAA was converted to ABL with 72.9% selectivity.
Embodiment 3
[0081] MAA was treated sequentially with EO (17.8 g, 1.0 eq.) and 1,8-diazabicyclo[5.4.0]-7-undecene (DBU) (62.5 g, 1.0 eq.) at 5 °C (46.9 g 0.40 mol) in MeOH (144.0 g). The reaction mixture was heated to 60° C. within 0.5 hours and maintained at this temperature for 2.5 hours. Quantitative GC analysis showed that 59.3% of MAA was converted to ABL with 82.2% selectivity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com