Mycobacterium bovis infection detection kit meditated by recombined fusion protein and method thereof
A technology for the detection of Mycobacterium bovis and infection, applied in the field of detection of Mycobacterium bovis infection, can solve the problems of poor specificity, complex antigenic components, and low sensitivity of serological detection methods, and achieve strong specificity and sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Construction of embodiment 1 recombinant plasmid pET-E6-M63-H65
[0028] 1.1 Extraction of Mycobacterium bovis genomic DNA
[0029] Refer to the method described in the instructions of the Bacterial Genomic DNA Small Amount Rapid Extraction Kit (purchased from Beijing Biotech Gene Technology Co., Ltd.).
[0030] 1.2 Design of primers
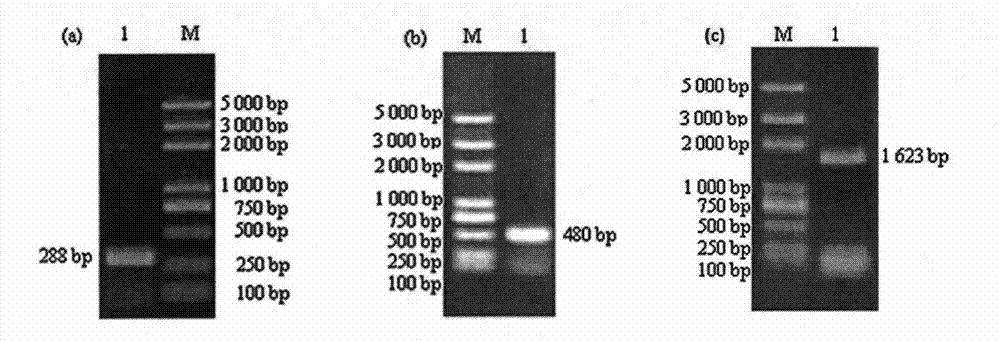
[0031] Specific primers were designed according to the ESAT-6, MPB63 and HSP65 gene sequences of Mycobacterium bovis genomic DNA (accession number BX248333) in GenBank. The primers were synthesized by Shanghai Sangon Biotechnology Co., Ltd., and the sequences are shown in Table 1 (the underline is the enzyme cut point, the shaded place is Linker).
[0032] Table 1PCR primer name, sequence and size of amplified product
[0033]
[0034] 1.3 PCR amplification of target gene and product recovery
[0035] Using Mycobacterium bovis genomic DNA as a template, LATaq DNA polymerase was used to amplify ESAT-6, MPB63 and HSP65 genes respecti...
Embodiment 2
[0048] Example 2 Expression and Purification of Mycobacterium bovis Recombinant Fusion Protein rE6-M63-H65
[0049] 2.1 Induced expression, purification, SDS-PAGE and Western-blot analysis of recombinant fusion protein
[0050] Transform the recombinant plasmid pET-E6-M63-H65 prepared in Example 1 into BL21(DE3) competent cells, pick a single colony and inoculate it into 200 mL of LB medium containing a final concentration of 25 μg / ml ampicillin, and shake at 37°C Bed culture until OD600=0.7, add IPTG with a final concentration of 1mM, induce culture at 30°C, 160rpm shaker for 10h, centrifuge at 9000rpm for 10min to collect the bacteria, resuspend the bacteria in 60mL PBS (pH 7.4), and sonicate the bacteria in an ice bath After crushing, the mixture was centrifuged at 12000rpm and 4°C for 30min, then the supernatant was taken, and the His Link TM Protein Purification Resin (purchased from Beijing Zeping Technology Co., Ltd.) operating manual to purify the recombinant protein...
Embodiment 3
[0052] The establishment of embodiment 3 Mycobacterium bovis detection method
[0053] 3.1 Collection and culture of bovine whole blood to be tested
[0054] ①Collect 5ml of bovine heparin anticoagulated whole blood under aseptic conditions, transport it to the laboratory at room temperature (22±5°C) and culture it within 8 hours after blood collection. ② Add anticoagulant blood to 24-well tissue culture plate, 1.5ml / well, 2 wells for each cow to be tested, then aseptically add 50μl Tris-Cl (100mM, pH 8.0, as negative control stimulus), 50μl containing rE6-M63-H65 solution (20μg) into the corresponding well, mix well, 37 ℃ CO 2 Incubate in the incubator for 24h. ③ After incubation, carefully draw 400 μl of the upper layer of plasma and transfer it to a 1.5ml centrifuge tube (plasma can be stored at 2-8°C for 7 days, and at -20°C for several months), and draw 50 μl of plasma from each tube as the sample to be tested ELISA detection of bovine IFN-γ release level.
[0055] 3....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com