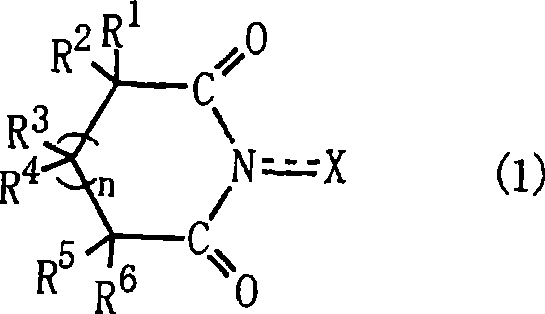
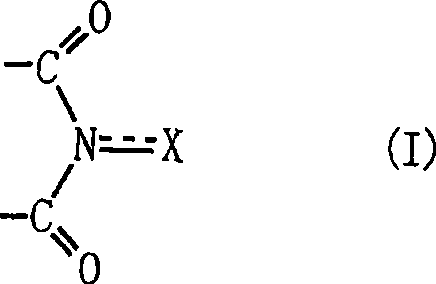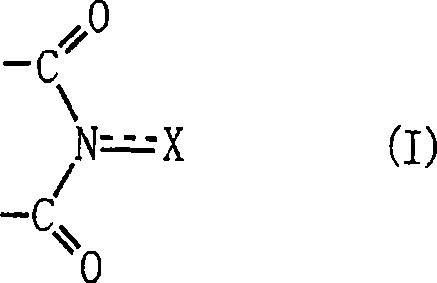Method for producing oxidation product of cycloalkane
一种氧化产物、环烷烃的技术,应用在过氧化合物制备、有机化合物的制备、烃氧化制备含氧化合物等方向,能够解决制备成本增加等问题,达到防止低聚化、减轻负荷、提高生成比的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0121] 5% by weight of N-hydroxysuccinyl obtained by adding 80 g (1.14 mol) of cyclopentane and dissolving 100 mg of N-hydroxysuccinimide in a 350 ml SUS316 autoclave equipped with a stirrer at room temperature (25°C) 2 g of imine aqueous solution was sealed in the autoclave, pressurized to 3 MPa (gauge pressure) with a mixed gas of 50% by volume of oxygen and 50% by volume of nitrogen, and stirred at 150° C. for 1 hour. As a result, the cyclopentane of 39mmol reacted (conversion rate 3.4%), and generated 9.4mmol cyclopentanone (selectivity 24.1%), 4.8mmol cyclopentanol (selectivity 12.3%), 12.5mmol cyclopentyl hydroperoxide ( selectivity 32.1%). The overall yield of cyclopentanone, cyclopentanol and cyclopentyl hydroperoxide was 2.3%.
Embodiment 2
[0123] 5% by weight of N-hydroxysuccinyl obtained by adding 80 g (0.95 mol) of cyclohexane and dissolving 100 mg of N-hydroxysuccinimide in a 350 ml SUS316 autoclave equipped with a stirrer at room temperature (25°C) 2 g of imine aqueous solution was sealed in the autoclave, pressurized to 3 MPa (gauge pressure) with a mixed gas of 50% by volume of oxygen and 50% by volume of nitrogen, and stirred at 150° C. for 1 hour. As a result, the cyclohexane of 36mmol reacted (conversion rate 3.8%), generated 9.2mmol cyclohexanone (selectivity 25.6%), 4.6mmol cyclohexanol (selectivity 12.8%), 12.3mmol cyclohexyl hydroperoxide (selectivity rate 34.2%). The overall yield of cyclohexanone, cyclohexanol and cyclohexyl hydroperoxide was 2.7%.
reference example 1
[0132] Example 1 was repeated several times, these reaction liquids were mixed, and the organic phase obtained by liquid separation was separated to obtain a cyclopentyl hydroperoxide-containing liquid used below. The composition of the liquid is as follows: 97.3% by weight of cyclopentane, 0.88% by weight of cyclopentanone, 0.41% by weight of cyclopentyl alcohol, 1.20% by weight of cyclopentyl hydroperoxide, and 0.21% by weight of esters (cyclopentyl esters converted to cyclopentyl esters). Pentanol is 0.12 wt%) and N-hydroxysuccinimide is 13 wtppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com