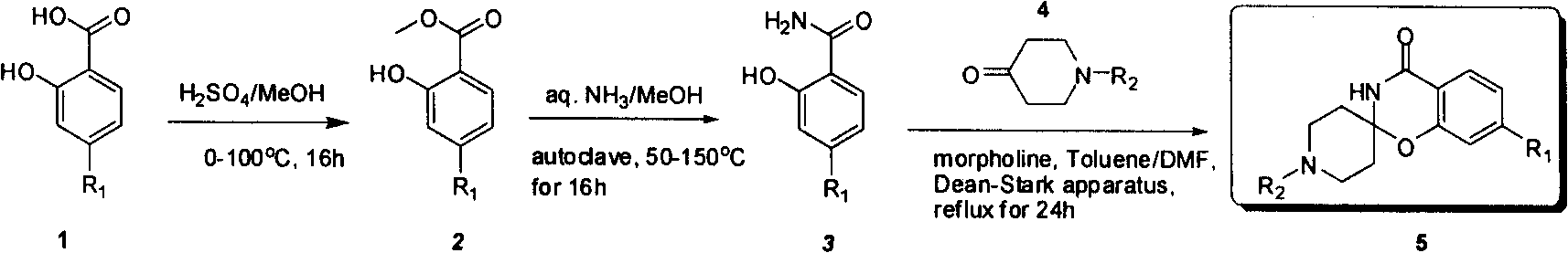
Industrial compounding method of mule (benzo (e) (1,3) oxazine-2, 4'-piperidine)-4(3H)-ketonic
A synthesis method and technology of piperidone, applied in the field of industrial synthesis of mule[benzo[e][1,3]oxazine-2,4'-piperidin]-4(3H)-one, can solve the problem of Incapable of large-scale production, many impurities and other problems, to achieve the effect of easy reaction, reasonable choice of reaction process, and low preparation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Synthesis of 4-aminomethyl salicylate
[0018] Add methanol (18.5L) to 4-aminosalicylic acid (3.7kg), stir for 15 minutes, cool to 10-15°C, slowly add concentrated sulfuric acid (3.7L), heat, reflux for 20-24 hours, and cool to 10- Stir at 15°C for 2-3 hours, filter, add ethyl acetate (15 L) and water (15 L) to the obtained solid, and add concentrated ammonia water to adjust the pH value to 8-9. Stir, stand still, separate liquid. The aqueous phase was extracted twice more with ethyl acetate (10 L×2). The organic phases were combined and concentrated to dryness to obtain 3.2 kg of product, yield: 80%. 1 H NMR (400MHz, DMSO-d 6 ): δ10.71(s, 1H, OH), 7.40(d, J=8.8Hz, 1H), 6.08(s, 2H, NH), 6.06(dd, J=8.4Hz, 1H), 5.94(d, J = 2.0 Hz, 1H), 3.73 (m, 3H); Ms (M+1, 168.1).
[0019] Synthesis of 4-amino salicylamide (aqueous ammonia)
[0020] Add methyl 4-aminosalicylate (700g) to a 10L autoclave, add methanol (3.5L) and concentrated ammonia water (3.5L), seal and heat,...
Embodiment 2
[0024] Synthesis of 4-amino salicylamide (ammonia)
[0025] Add 4-aminosalicylic acid methyl ester (130g) into a 10L autoclave, add saturated ammonia methanol solution (7.5L), seal and heat, stir at 125°C for 16 hours, cool down, and concentrate to dryness. Methanol (200 mL) was added, filtered, and washed with dichloromethane (300 mL). Put into a vacuum oven and dry for 12 hours to obtain 70 grams of product, yield: 55%. Its test data is as shown in above-mentioned embodiment 1.
[0026] Synthesis of 7-aminospiro[benzo[e][1,3]-2,4'-Boc-piperidin]-4(3H)-one (old process, p-toluenesulfonic acid catalytic)
[0027] 4-Amino salicylamide (30 grams) was added into a 2L there-necked flask, then N, N-dimethylformamide (200mL), toluene (400mL), p-toluenesulfonic acid (47.3 grams), N- Boc-4-piperidone (1050 g) and 4 Molecular sieves (10 g). Heated to 115°C, carried water through toluene, and heated to reflux for 48 hours. The temperature was lowered, and the pH was adjuste...
Embodiment 3
[0029] Synthesis of 4-aminomethyl salicylate
[0030] Add methanol (3L) to 4-aminosalicylic acid (300g), stir for 15 minutes, cool to 0-10°C, slowly add thionyl chloride (300g), return to room temperature, stir overnight, filter, and add acetic acid to the obtained solid Ethyl ester (3000mL) and water (3000mL), and concentrated ammonia water was added to adjust the pH value to 8-9. Stir, stand still, separate liquid. The aqueous phase was extracted twice more with ethyl acetate (2000 mL×2). The organic phases were combined and concentrated to dryness to obtain 270 g of the product with a yield of 72.9%. Its test data is as shown in above-mentioned embodiment 1.
[0031] Synthesis of 4-amino salicylamide (aqueous ammonia)
[0032] Add 4-aminosalicylic acid methyl ester (270g) to a 10L autoclave, add tetrahydrofuran (3L) and concentrated ammonia water (3L), seal and heat, stir at 100°C for 20 hours, cool down, and concentrate to dryness. Dichloromethane (3.5 L) was adde...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com