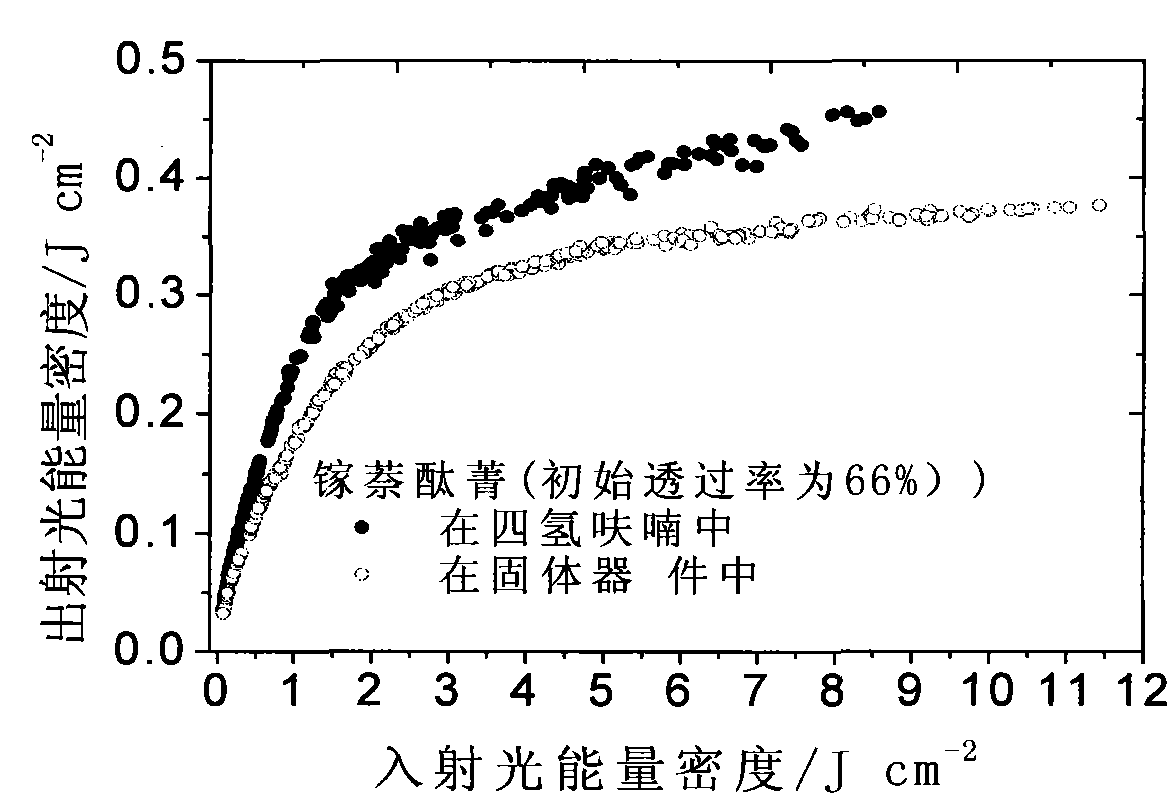
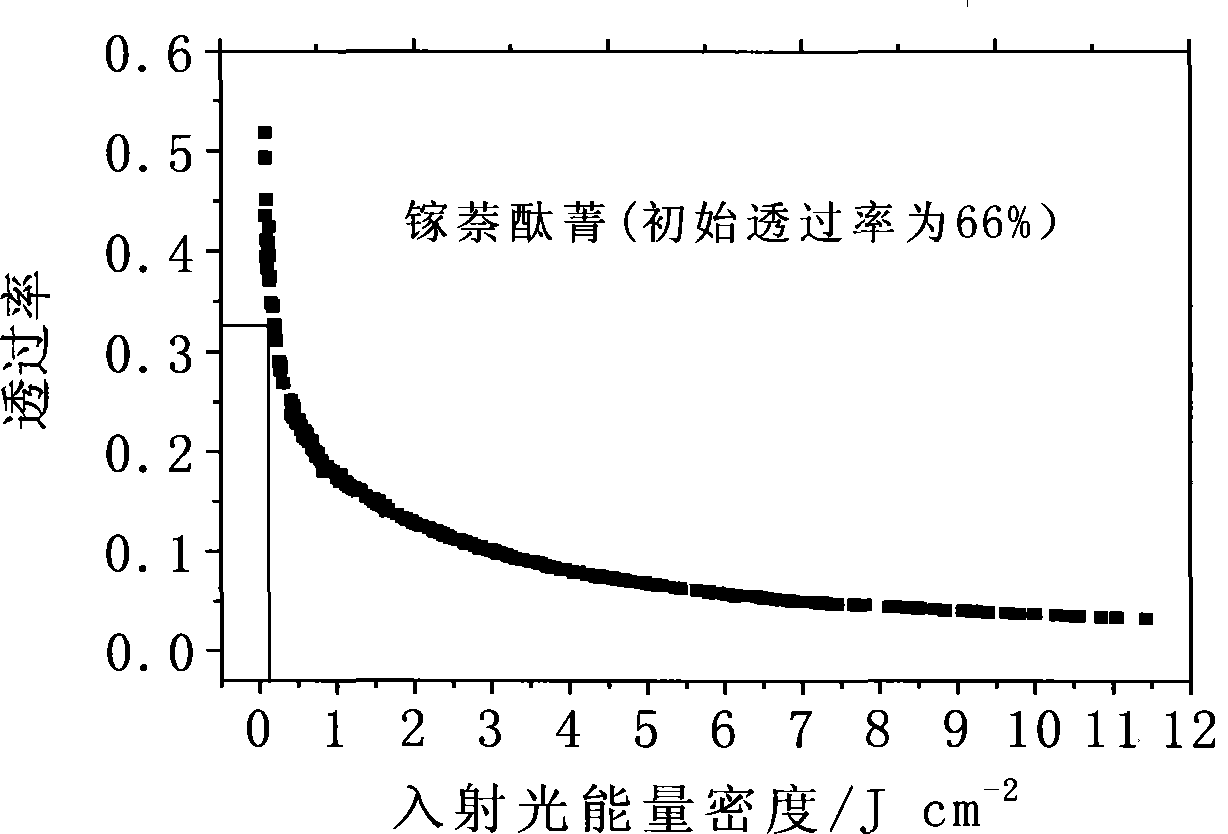
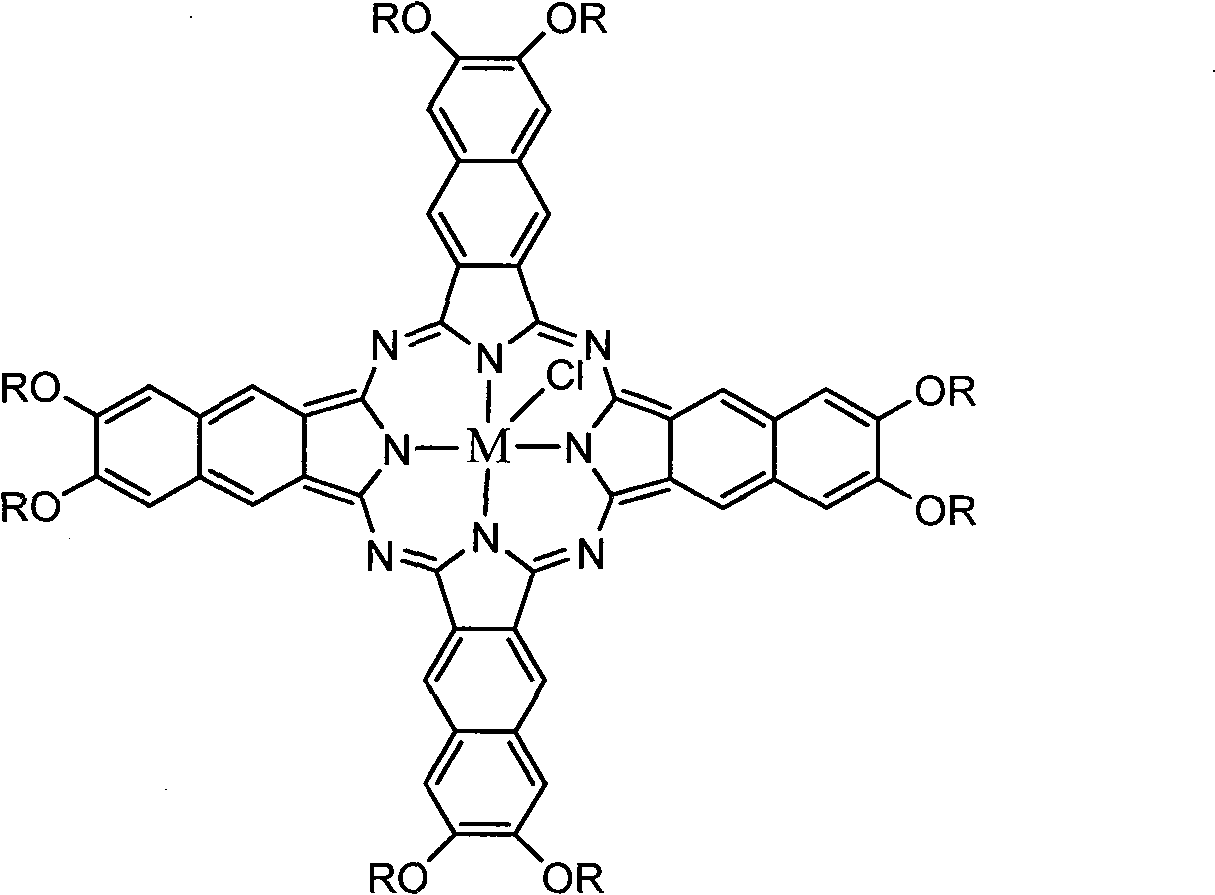
Solid naphthalocyanine device with optical limiting properties
A naphthalocyanine, optical limiting technology, applied in instruments, optics, nonlinear optics, etc., can solve problems such as fragile anti-laser ability, and achieve good anti-laser ability, good transparency, and good anti-laser ability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Add 4.74g (10mmol) of 6,7-di-tert-butylphenoxy-2,3-naphthalene dinitrile, 10ml of analytically pure n-pentanol to a 50ml three-necked flask, heat the oil bath to 60°C, and add 1ml of 1 , 8-diazacyclo[5,4,0]undecene-7 (DBU) was used as a catalyst, and after heating slowly to 110°C for 2 hours, 0.45 g of anhydrous GaCl was added 3 And the temperature was raised to 140°C, heated to reflux and stirred for 36 hours, then stopped heating, and when the solution was cooled to room temperature, 50ml of a mixed solution of methanol and water with a volume ratio of 1:1 was added, filtered and washed to obtain yellow-green crude The product was passed through a silica gel column with a mixed solvent of dichloromethane and tetrahydrofuran (THF) at a volume ratio of 20:1 as a developing solvent to obtain a gallium naphthalocyanine compound with a yield of 62%. UV-Vis (THF) λmax: 799, 340nm; Mass Spectrum (MALDI-TOF): 2001.1 (M + ), 1966.2 (M + -Cl), 1817.1 (M + -Cl-OR), 1669.0 (M ...
Embodiment 2
[0028] Add 4.74g (10mmol) of 6,7-di-tert-butylphenoxy-2,3-naphthalene dinitrile and 10ml of analytically pure n-pentanol into a 50ml three-necked flask, heat the oil bath to 60°C, and then add 1ml of DBU As a catalyst, after heating slowly to 110°C for 2 hours, add 0.55 g of anhydrous InCl 3 And the temperature was raised to 140°C, heated to reflux and stirred for 36 hours, then stopped heating, and when the solution was cooled to room temperature, 50ml of a mixed solution of methanol and water with a volume ratio of 1:1 was added, filtered and washed to obtain a yellow-green compound The product was passed through a silica gel column with a mixed solvent of dichloromethane and tetrahydrofuran at a volume ratio of 20:1 as a developing solvent to obtain an indium naphthalocyanine compound with a yield of 58%. UV-Vis (THF) λmax: 802, 339nm; Mass Spectrum (MALDI-TOF): 2046.2 (M + ), 2010.6 (M + -Cl), 1861.5 (M + -Cl-OR); 1 H-NMR (DMSO-d6, 400Hz): 9.17(s, 8H), 8.41(s, 8H), 7.5...
Embodiment 3
[0030] 4.74g (10mmol) of 6,7-di-tert-butylphenoxy-2,3-naphthalene dinitrile, 0.46g of anhydrous SnCl 2 10ml of 1-chloronaphthalene was added to a 50ml three-necked flask, and the oil bath was heated to 190°C, and the reaction was stopped after 4 hours of reaction. When the solution was cooled to room temperature, 50ml of n-hexane was added, filtered and washed to obtain yellow-green The group product was obtained by using a mixed solvent of dichloromethane and tetrahydrofuran with a volume ratio of 20:1 as a developing solvent to pass through a silica gel column to obtain a tin naphthalocyanine compound with a yield of 55%. UV-Vis (THF) λmax: 821, 346nm; Mass Spectrum (MALDI-TOF): 2085.6 (M +), 2014.5 (M + -2Cl), 1485.8 (M + -4OR); 1 H-NMR (CDCl 3 , 400Hz): 9.54(s, 8H), 8.55(s, 8H), 7.50-7.55(d, 16H), 7.27-7.33(d, 16H), 1.44(s, 72H); elemental analysis (%): measured Values C 73.64, H 5.75, N 5.37; found C 73.44, H 5.04, N 5.83.
[0031] 2. Fabrication of Solid Devices ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com