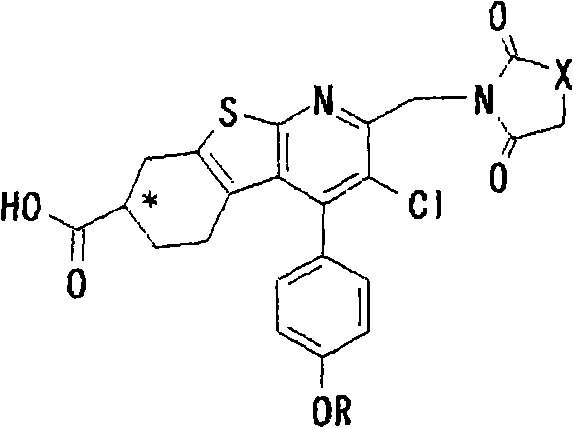
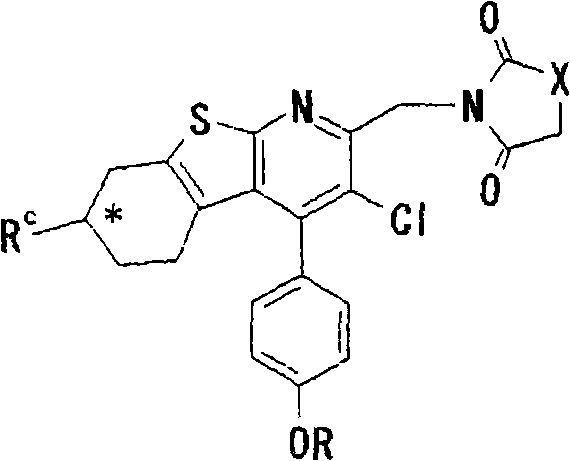
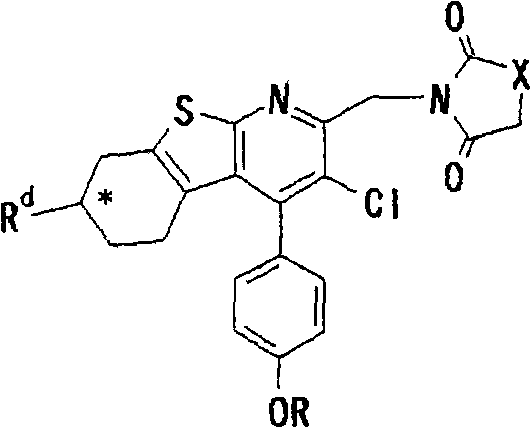
Thienopyridine derivatives, production method and use thereof
A compound and mixture technology, applied in organic chemistry methods, chemical instruments and methods, drug combinations, etc., can solve problems such as long-term use shortage, arthritis ineffectiveness, side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0289] The present invention is described in more detail below by the following reference examples, examples, preparation examples and experimental examples, these reference examples, examples, preparation examples and experimental examples should not be construed as limiting, and can The scope of the premise varies.
[0290] Unless otherwise stated, the elution of column chromatography in Reference Examples and Examples was performed under observation of TLC (thin layer chromatography). TLC observation using silica gel 60F produced by Merck 254 A solvent that was used as a TLC plate and eluted using column chromatography was used as a developing solvent. Detection utilizes a UV detector. Silica gel 60 (70-230 mesh size) produced by Merck was used as silica gel for column chromatography. The room temperature here generally refers to a temperature of about 10°C to 35°C. In addition, sodium sulfate or magnesium sulfate is used to dry the extract.
[0291] Abbreviations used...
reference example 1
[0344] 4-Methoxybenzoylacetonitrile
[0345]
[0346] Sodium methoxide (3.046 kg) and acetonitrile (2.135 kg) were added to a dimethyl sulfoxide solution (21.6 L) of methyl 4-methoxybenzoate (7.2 kg), and the mixture was stirred at 110° C. for 2 hours . Water (10.83 L) was added dropwise at not exceeding 15°C, and acetonitrile (14.4 L) was added. Then 6N HCl was added to adjust the pH to 7.9 without exceeding 20°C, and the mixture was extracted with ethyl acetate (72 L). The aqueous layer was further extracted with ethyl acetate (36.32 L). The organic layers were combined and concentrated until the weight of the concentrate was 17.39 kg. Methanol (17.84 L) was added to the mixture, and then water (17.84 L) was added dropwise. The mixture was stirred at 5°C for 1 hr, and the precipitated crystals were collected by filtration and washed with methanol-water (1:1) to obtain the title compound (6.40 kg, 82.7%).
[0347] 1 H-NMR (CDCl 3 ) δ; 3.90 (3H, s), 4.03 (2H, s), 6.9...
reference example 2
[0349] 2-Amino-4,5,6,7-tetrahydro-3-(4-methoxybenzoyl)-1-benzothiophene-6-carboxylic acid ethyl ester
[0350]
[0351]4-Methoxybenzoyl acetonitrile (13.6g), cyclohexanone-4-carboxylic acid ethyl ester (14.0g), sulfur (2.7g), morpholine (7.3g) obtained in Reference Example 1 And ethanol (300ml) was stirred under reflux for 3 hours. After the reaction was completed, the reaction solution was concentrated under reduced pressure. The obtained residue was purified by column chromatography (developing solvent: ethyl acetate-hexane (1:1, v / v)), and the title compound (25.0 g , 87%). Melting point: 102-103°C.
[0352] 1 H-NMR (CDCl 3 )δ; 1.26 (3H, t, J=7.0Hz), 1.50-1.70 (1H, m), 1.87-2.18 (3H, m), 2.61-2.87 (3H, m), 3.86 (3H, s), 4.15 (2H, q, J = 7.0 Hz), 6.30 (2H, brs), 6.90 (2H, d, J = 8.8 Hz), 7.51 (2H, d, J = 8.8 Hz).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com