Method for preparing 5-aza-2'-deoxycytidine and its intermediates
A technology of deoxycytidine and azacytosine is applied in the field of preparing the new azacytosine deoxynucleoside and the intermediate field of preparing 5-aza-2'-deoxycytidine, and the process is simple and reproducible. Good performance and low content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
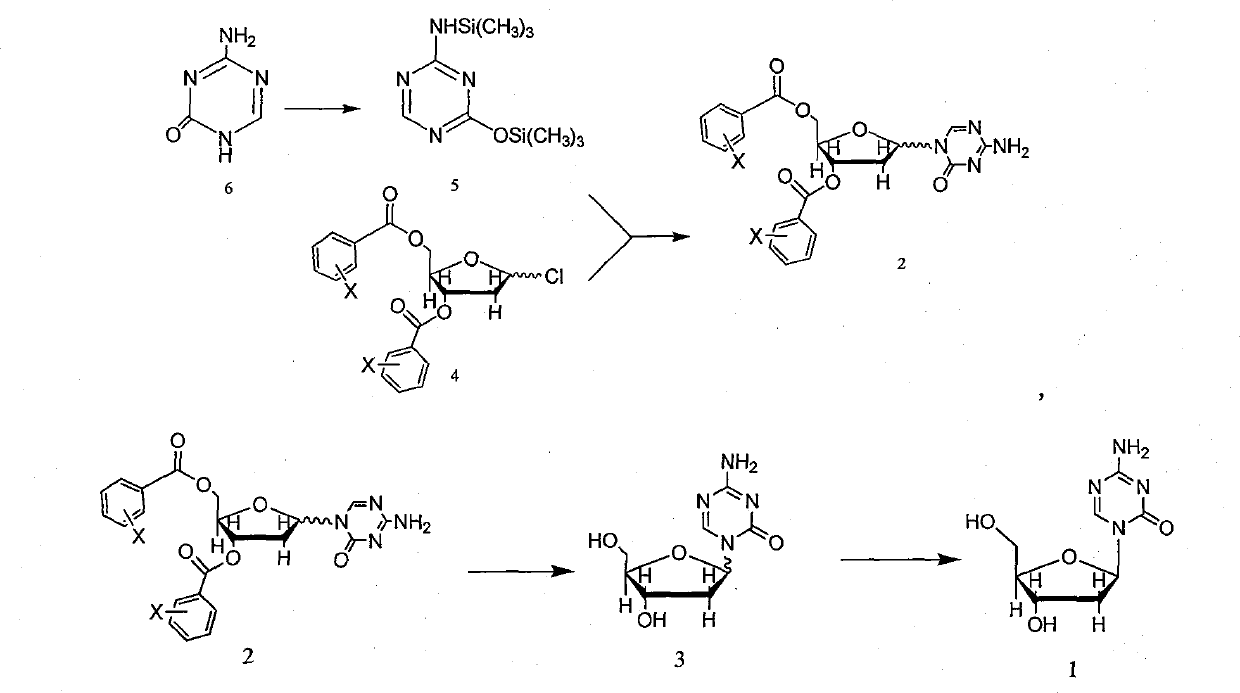
[0043] Preparation of 1-(2'-deoxy-3',5'-di-O-p-chlorobenzoyloxy-D-arabinofuranosyl)-5-azacytosine Weigh 5-azacytosine 313.6g, put in a reaction bottle, add 8L of hexamethyldisilazane, stir, add 200ml of trimethylchlorosilane, protect with nitrogen, heat to reflux, react for 24 hours, the raw materials are dissolved, the reaction is completed, heat and evaporate to dryness under reduced pressure Solvent, obtain the 5-azacytosine of silanization, do not need to deal with and carry out the following reaction continuously.
[0044] Add silanized 5-azacytosine to 16L of 1,2-dichloroethane, stir to dissolve, add 1-chloro-3,5-di-O-p-chlorobenzoyl-2-deoxy-D-ribose 395.6g (0.92mol), cooled to 5°C, temperature-controlled addition of a mixture of 333.6ml of trimethylsilyl trifluoromethanesulfonate (TMSOTf) and 2L of acetonitrile, and reacted at room temperature for 3.5h.
[0045] Pour the reaction solution into 30L of cold water, extract twice with dichloromethane, 20L each time, combin...
Embodiment 2
[0051] Preparation of 5-aza-2'-deoxycytidine
[0052] Put 100L of methanol in a reaction flask, add 16 grams of sodium methoxide, stir, and add 1-(2'-deoxy-3',5'-di-O-p-chlorobenzoyloxy-D in Example 1 -Arabinofuranosyl)-5-azacytosine 510 grams, reacted at room temperature for 20 hours, concentrated to an appropriate volume, left to cool, filtered, washed with methanol, and dried in vacuo to obtain 150 grams of white crystal product, the related substances determined by HPLC were less than 1.5% .
[0053] Methanol was recrystallized again, as determined by HPLC, the related substance was 0.5%.
[0054] mp: 191.1-193.2;
[0055] [α] D 22 =+68.1° (30min), +57.8° (6hr) (c=0.5, H2O).
[0056] MS[M+H]+: 228.09;
[0057] Elemental analysis C8H12N4O4: theoretical C42.10, H5.30, N, 24.55, measured C42.02, H5.33, N, 24.35;
[0058] 1HNMR test (DMSO-d6) δ: 2.13~2.21(m, 2H), 3.54-3.61(m, 2H), 3.82(t, 1H),
[0059] 4.24 (m, 1H), 5.01 (m, 2H), 5.20 (d, 1H), 6.03 (t, 1H), 7.47 (s, 2H...
Embodiment 3
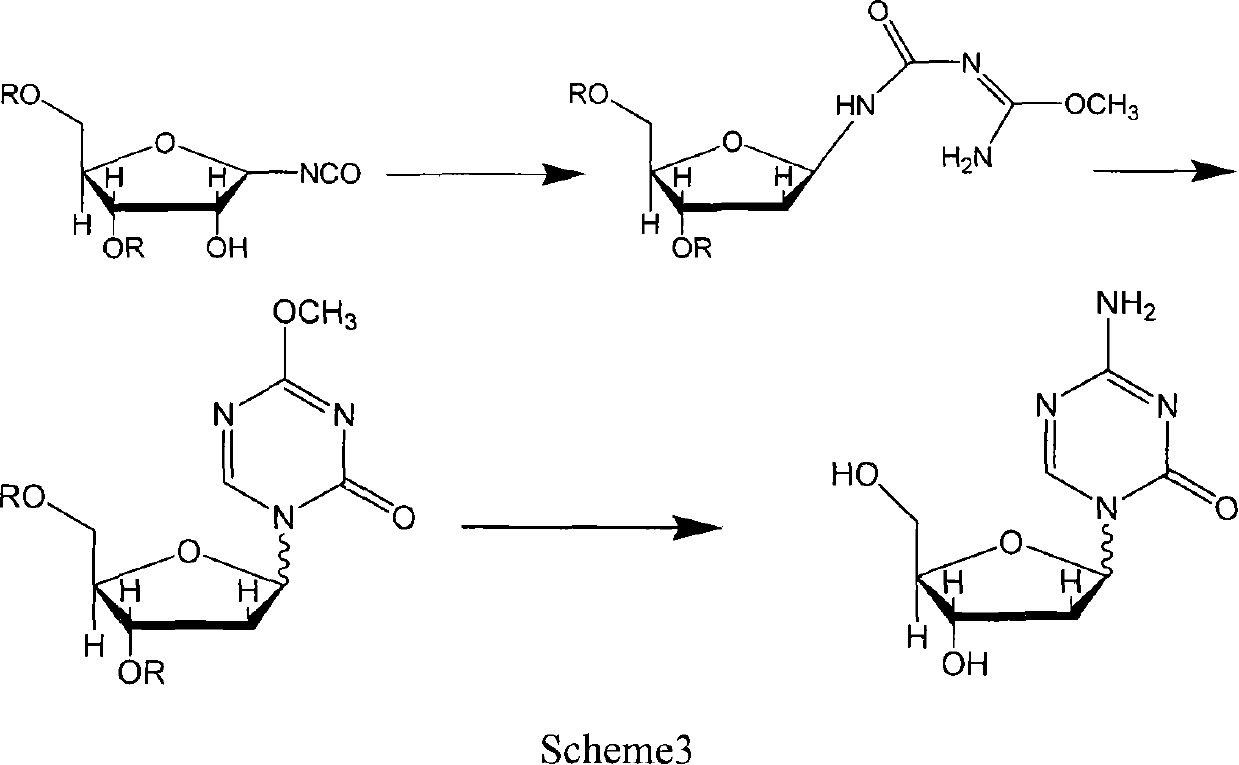
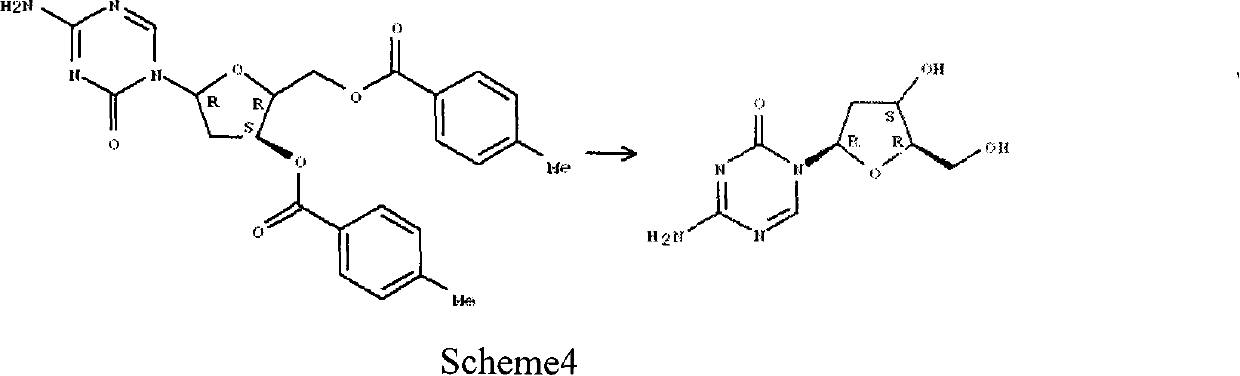
[0062] Preparation of α-isomer of 2'-deoxy-5-azacytosine nucleoside
[0063] The mother liquor of 5-aza-2'-deoxycytidine prepared by the method of Reference Example 2 was subjected to column chromatography (gradient elution of chloroform-methanol system), and the fraction containing α-isomers was collected and recrystallized from a mixed solvent of methanol / ether to obtain The alpha isomer of 5-aza-2'-deoxycytidine was used as white powder crystals.
[0064] 1HNMR (DMSO-d6): 8.26 (s, 1, H6), 7.4 (e, 2, NH2), 5.94 (d, 1, Hl'), 5.21 (d, 1, OH3'), 4.83 (t, 1 , OH5'), 4.25(m, 2, H3', 4'), 3.4(m, 2, H5'), 2.3(m, 2, H-2');
[0065] Mp: Decompose at 181-182°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com