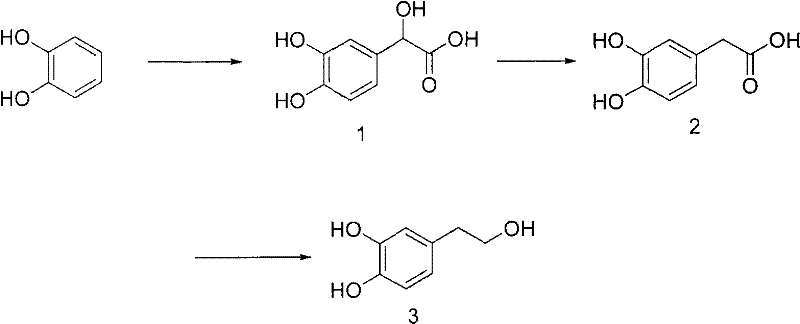
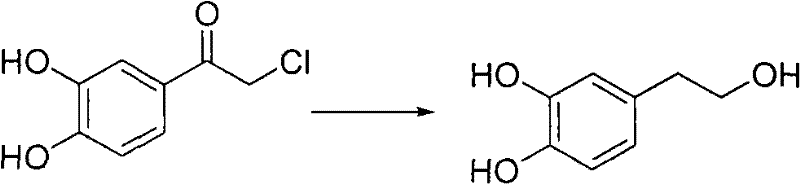
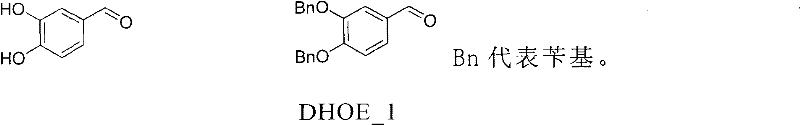Synthesis method of 3, 4-dihydroxy phenylethanol
A technology of dihydroxyphenylethanol and a synthetic method, which is applied in 3 fields, can solve problems such as low product quality and yield, short heating time of the extract, and difficulty in obtaining acetophenone, etc., to achieve reasonable reaction steps and design of reaction steps Reasonable and easy to purify effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] 3,4-dihydroxybenzaldehyde (13.81g, 0.1mol), benzyl chloride (31.6g, 0.25mol), anhydrous potassium carbonate (69g, 0.5mol), DMF (200ml) were added to the reaction flask, and the temperature was raised To 80°C, react for about 4h, TLC detection, then filter, recover DMF and residue, add a small amount of water, stir to obtain a yellow solid product, and then recrystallize with ethanol to obtain DHOE_1 (28.1g, 88%), mp: 90 -91°C.
[0044] Put DHOE_1 (100g, 314mmol) in a 1000ml reaction flask, add 300ml methanol, heat up to 25°C, then add sodium methoxide (45g, 55%), keep dropping methyl chloroacetate (51g, 471mmol) below 20°C , Calculate the time from the dropwise addition, and react at 17-20°C for 4 hours to epoxidize. Then at 20-25°C, 112g of liquid caustic soda and methanol mixture (liquid caustic soda:methanol=1:2) was added dropwise, and the time was counted from the dropwise addition, and reacted for 3 hours to hydrolyze the epoxy. Under stirring again, 118g of 35%...
Embodiment 2
[0048] Add 14g of 3,4-dihydroxybenzaldehyde, 32g of benzyl bromide, 70g of anhydrous potassium carbonate, and 200ml of DMF into the reaction flask, raise the temperature to 20°C, react for about 24h, detect by TLC, and then filter to recover DMF and the residue. A small amount of water was added and stirred to obtain a yellow solid product, which was then recrystallized from ethanol to obtain DHOE_128.1g, yield 88%, mp: 90-91°C.
[0049] 100gDHOE_1 is put into the reaction flask, add 300ml methyl alcohol, be warming up to 25 ℃, add 45g sodium ethylate (55%) again, keep on the temperature and add dropwise 50g methyl chloroacetate when 10 ℃, start to calculate time from dropwise, in React at 10°C for 10 hours to epoxidize. Then at 10-20°C, 112g of liquid caustic soda and methanol mixture (liquid caustic soda:methanol=1:2) was added dropwise, and the time was counted from the beginning of the dropwise addition, and reacted for 6 hours to hydrolyze the epoxy. Under stirring again...
Embodiment 3
[0053] Add 14g of 3,4-dihydroxybenzaldehyde, 32g of benzyl chloride, 70g of anhydrous potassium carbonate, and 300ml of DMF into the reaction flask, raise the temperature to 60°C, react for about 12h, detect by TLC, and then filter to recover DMF and the residue. A small amount of water was added and stirred to obtain a yellow solid product, which was then recrystallized from ethanol to obtain DHOE_129.2g, yield 90%, mp: 90-91°C.
[0054] Put 100g of DHOE_1 into the reaction flask, add 300ml of methanol, raise the temperature to 25°C, then add 45g of sodium methoxide (55%), keep it below 15°C, add 50g of methyl chloroacetate dropwise, calculate the time from the drop, at 15°C The reaction was carried out for 6 hours to epoxidize. Then dropwise add liquid caustic soda and methanol mixed solution 112g (liquid caustic soda:methanol=1:2) at 20-25°C, count the time from the dropwise addition, react for 4 hours, and make the epoxy compound hydrolyze. Under stirring again, 118g of 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com