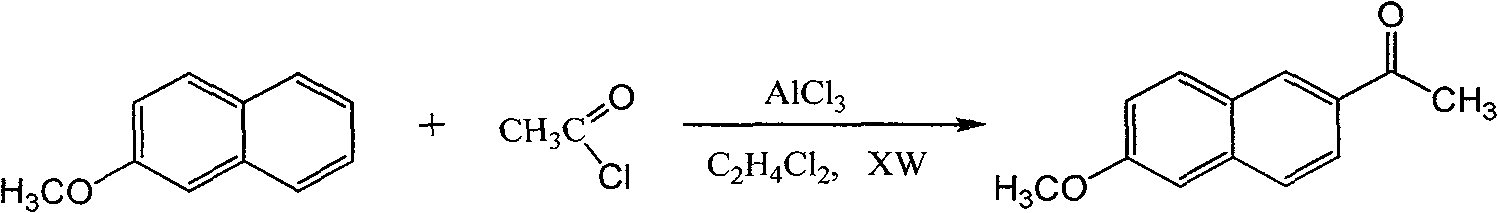Method for preparing 6-methoxy-2-acetonaphthalene
A methoxy and acetyl technology, applied in the field of medicine and chemical industry, to achieve the effect of reasonable reaction steps, less environmental pollution and high quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
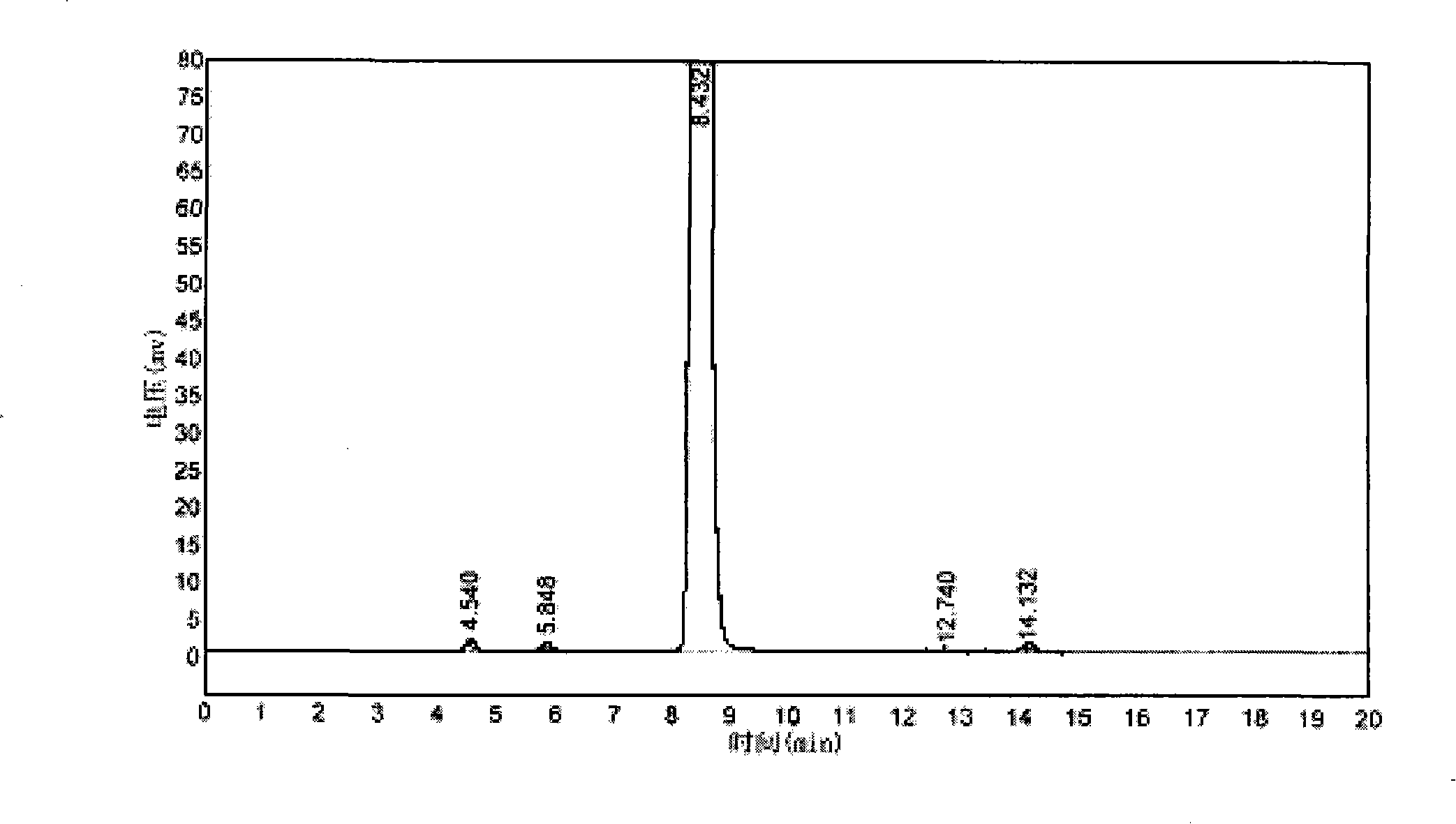
[0029] Add 300ml of 1,2-dichloroethane, 80g of β-naphthyl methyl ether, and 9ml of nitroethane into a 500ml three-neck flask equipped with a stirrer, a thermometer, and a reflux cooler. After stirring and dissolving, cool down to 5°C~ 8°C, put in 84g of aluminum trichloride, and after dissolving, add 44g of acetyl chloride dropwise at a temperature of 10°C to 14°C. Incubate for 20 hours under the condition of 36-38° C., the product is 92.5% and the isomer is 0.8%.
[0030]Pour the above reaction liquid into crushed ice water for ice thawing, control the temperature of the reaction liquid before ice thawing to be 36-38°C, let it stand for stratification, wash the water layer with 1,2-dichloroethane once, and put the washed The organic layer was incorporated into the original organic layer, and the organic layer was distilled under normal pressure to remove the solvent 1,2-dichloroethane. After precipitating, distillation is carried out under the condition that the vacuum degre...
Embodiment 2
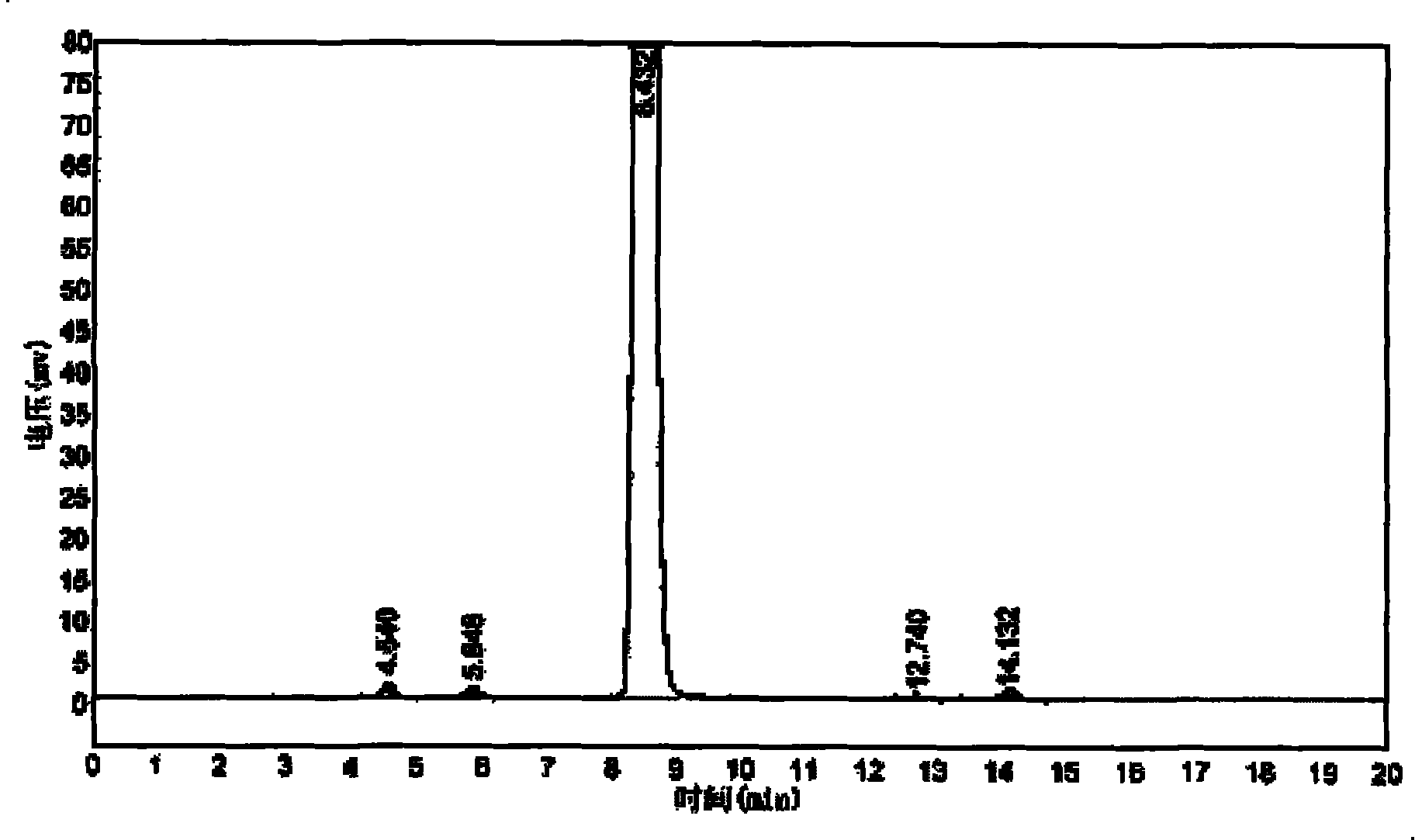
[0032] The reaction device is the same as in Example 1, and will not be repeated; add 450ml of 1,1-dichloroethane, 80g of β-naphthyl methyl ether, and 11ml of nitromethane, stir and dissolve, cool to 8°C to 10°C, and put in trichloride After dissolving 90g of iron, add 60g of acetyl chloride dropwise at a temperature of 15°C to 20°C. After the dropwise addition, the temperature rises slowly for 4 to 5 hours until the temperature reaches 45°C. After incubation for 18 hours, the product was sampled and detected to be 90.5%, and the isomer was 1.2%.
[0033] Pour the above reaction liquid into crushed ice water for ice thawing, control the temperature of the reaction liquid before ice thawing at 45-48°C, let it stand for stratification, wash the water layer with 1,1-dichloroethane once, and put the washed The organic layer was incorporated into the original organic layer, and the organic layer was distilled under normal pressure to remove the solvent 1,1-dichloroethane. After pr...
Embodiment 3
[0035] The reaction device is the same as in Example 1, and will not be repeated; add 300ml of 1,2-dibromoethane, 80g of β-naphthyl methyl ether, and 9ml of 1-nitropropane, stir and dissolve, cool to 3°C to 5°C, and put in chlorine After dissolving 92g of zinc chloride, add 56g of acetyl chloride dropwise at a temperature of 5°C to 10°C. After the dropwise addition, the temperature rises slowly for 3 to 4 hours until the temperature reaches 20°C. After 30 hours of incubation at high temperature, 85.3% of the product and 4.6% of isomers were detected by sampling.
[0036] Pour the above reaction liquid into crushed ice water for ice thawing, control the temperature of the reaction liquid before ice thawing at 20-25°C, let it stand for stratification, wash the water layer with 1,2-dibromoethane once, and put the washed The organic layer was incorporated into the original organic layer, and the organic layer was distilled under normal pressure to remove the solvent 1,2-dibromoeth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com